As the field of interventional cardiology has grown, so too has interest in mitigating severe adverse outcomes such as stroke. Unfortunately, the risk of stroke is increased by many underlying disease conditions (atrial fibrillation, patent foramen ovale, carotid artery disease and other vascular disease affecting brain perfusion, etc.), making clear differentiation between cardiac intervention-related cerebral infarction and the effect of other coexisting factors very difficult. In this regard, the prevalence of atrial fibrillation in average transcatheter aortic valve replacement (TAVR) patient cohorts has been reported to be over 31 % (9 studies, 285/902 patients)1–9 and the prevalence of previous stroke has been reported as high as 10.8 % (14 studies, 111/1028 patients),6–14 confounding the attribution of stroke to TAVR procedures.
Covert Central Nervous System Infarction
While clinically overt stroke is one of the most feared adverse events among patients undergoing invasive or interventional procedures, subclinical neurological events or covert central nervous system (CNS) infarctions are also a significant risk. In general, covert CNS infarctions are best detected by diffusion-weighted MRI in the absence of any clinically apparent symptoms. They are prevalent in approximately 20 % of the general population, but the risk and incidence rate increase drastically with age and in populations affected by certain medical conditions (see above), as well as following interventional and surgical procedures. The incidence of new lesions following TAVR, for instance, is estimated to be 68–91 %,15 with at least one study demonstrating evidence of new cerebral infarctions in 100 % of patients.16 As with clinically overt stroke, substantial evidence has shown that the presence of covert cerebral infarctions increases the risk of future stroke, cognitive decline, dementia and mortality. The same applies for surgical aortic valve interventions where even larger brain infarctions are seen.17–21
Stroke Burden
Stroke from any cause poses a tremendous strain on a patient, their family and the healthcare system. Hospitalisation due to stroke is costly, and continued expenditures including inpatient care, rehabilitation and long-term care exceed US $140,000 over a lifetime.22 Recent studies also conclude that these costs increase greatly with increased stroke severity and resulting disability.22,23
TAVR and Stroke
In the last 15 years, TAVR has emerged as an important therapeutic option for patients with severe aortic stenosis, particularly those at increased risk of operative mortality or morbidity with traditional surgical aortic valve replacement (SAVR).24–28 Although TAVR has consistently demonstrated similar or better outcomes than SAVR,24–29 neurological complications remain a concern. Early in the TAVR experience, reports of clinical stroke rates up to 5 %,24,28 mainly occurring within 1 week of the procedure, garnered significant attention, and have been shown to be associated with lower 1-year survival rates.11 As TAVR populations, procedures and devices have matured, reported rates of overt clinical stroke within controlled clinical trials,29 and in real-world practice, remain a critical health issue. Newer, repositionable TAVR devices allow for more precise valve implantation, but this may be at the expense of higher embolic burdens, increasing the risk for stroke and death.21 In fact, the STS/ACC Transcatheter Valve Therapy Registry (TVT Registry), designed to benchmark safety and efficacy outcomes related to US commercial TVT procedures (>53,000 cases to date), showed no significant decline in 30-day site reported stroke rates from 2012 (2.6 %) to 2015 (2.2 %) despite new evolutions in TAVR devices.11 The TVT Registry also highlighted that stroke risk is independent of increased physician TAVR experience. This finding is corroborated by a multinational registry of over 1,900 subjects in 80 international centres.30 It is important to emphasise that the TVT Registry collects information on site reported strokes, which does not require diagnosis of a stroke by a certified neurologist, thus likely underestimating the true stroke rate in the real-world setting. Further confounding the true stroke rate, the definition and classification of stroke has been controversial and has changed over the last decade.21
As mentioned above, procedure-related stroke or new ischemic cerebral infarctions may result from a variety of patient- and disease-related causes such as severity of atherosclerosis, age, gender, hyperlipidaemia, history of atrial fibrillation and/or technical aspects of the procedure itself, including mechanical manipulation of instruments or interventional devices. A rapidly growing body of clinical evidence shows that embolic debris is generated in the vast majority of patients undergoing TAVR.12 Debris can be up to 1 cm in size, is from a variety of vascular and valvular tissue sources, and has been captured during the use of a variety of different transcatheter valves and interventional approaches.1
Sentinel
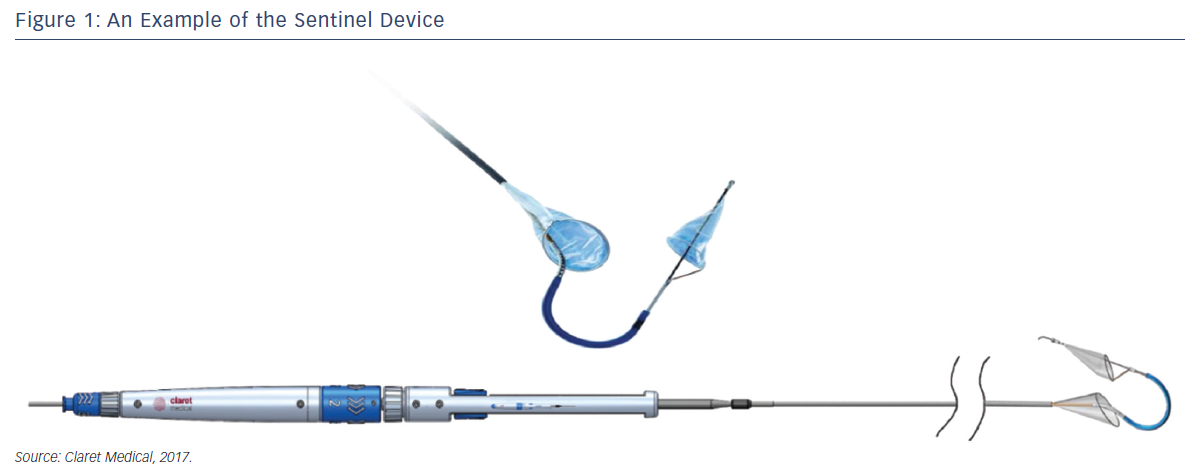
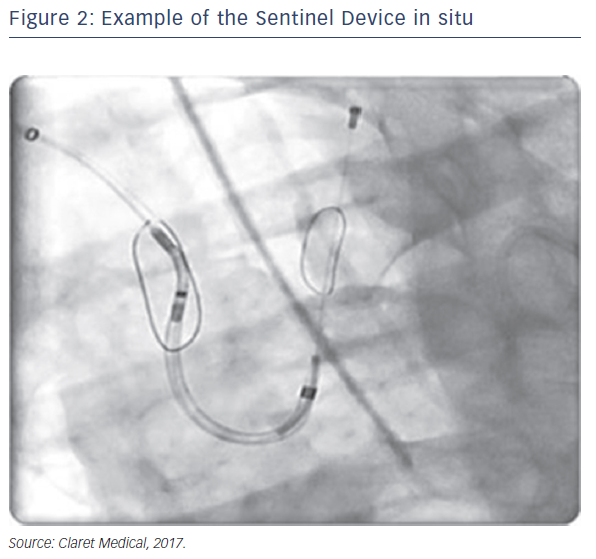
Sentinel™ Cerebral Protection System (Claret Medical, Santa Rosa, CA, USA) is designed to protect the brain from the risk of stroke by filtering, capturing and removing debris dislodged during many interventional and surgical left heart, as well as endovascular, procedures. The device consists of two filters within a single 6 French delivery catheter percutaneously placed from the right radial (preferred) or brachial artery over a 0.014" guide wire. The filters are deployed in the brachiocephalic and the left common carotid arteries, the two main arteries supplying the brain, and are withdrawn into the catheter at the conclusion of the procedure (Figures 1 and 2).
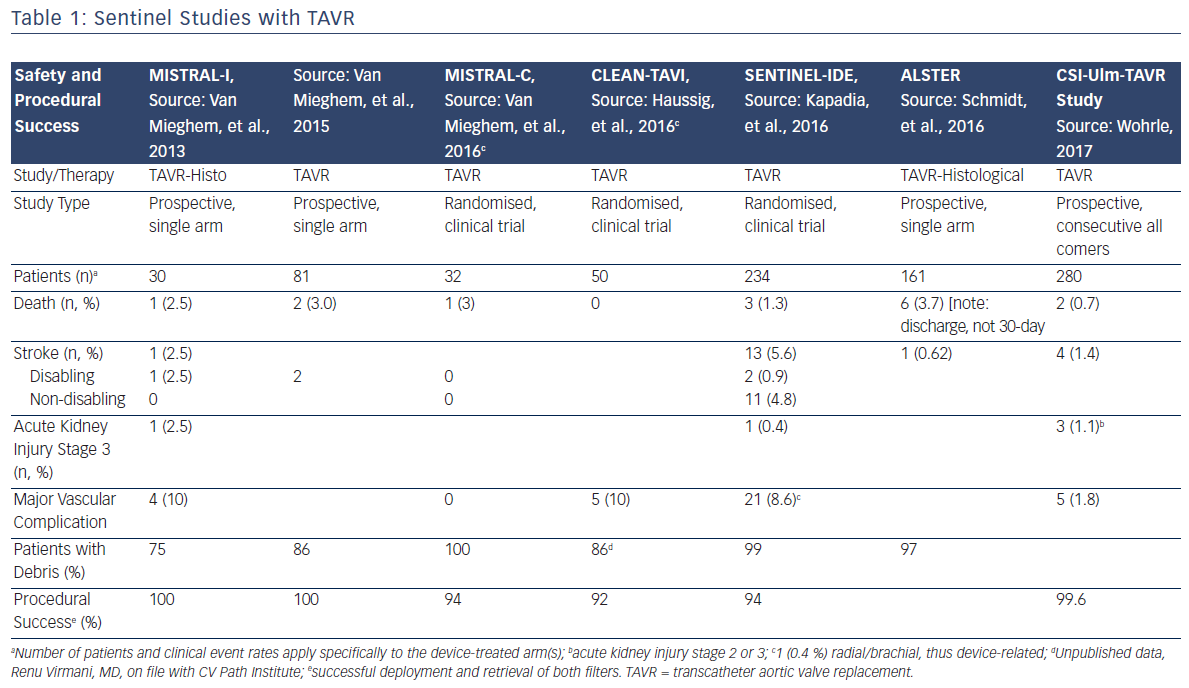
Since the initial proof of concept study in 2011, numerous manuscripts or presentations describing experience with Sentinel have been published. Sentinel has shown procedural success of greater than 90 % across multiple studies and therapies, which is paralleled by its strong safety profile (Table 1).1–3,12–15 Although MRI imaging evidence in terms of reduced lesion number and volume is variable across studies,2,3,13 detailed histopathological assessments have described near ubiquitous, and remarkably consistent, capture of a wide variety of debris types within the filters (Figure 2). Not surprisingly, due to the success experienced with Sentinel in TAVR procedures, other structural interventions known to be cardioembolic, including mitral valve interventions (MitraClip),31 left atrial appendage occlusion procedures (LAAO)32 and thoracic endovascular aneurysm repair (TEVAR),33,34 have shown remarkably similar patterns of debris capture and procedural success with the use of cerebral embolic protection (CEP).
Patient Population
The Sentinel device was originally conceived as an adjunctive therapy for TAVR; thus, the majority of published studies have been conducted in patients with severe, symptomatic aortic stenosis undergoing TAVR. To date, studies of Sentinel in conjunction with TAVR therapy have been conducted in similar patient populations, which makes comparisons and data pooling between studies possible. Indeed, although the current literature summary does not include any formal Sentinelspecific meta-analysis, a review and meta-analysis of cerebral embolic protection trials, including SENTINEL, has been published.35
Overall, patients included in studies of Sentinel with TAVR to date have been elderly and at increased risk of surgical mortality and morbidity (including perioperative stroke) based on traditional surgical risk scores such as the Society of Thoracic Surgeons and the Logistic EuroSCORE. Importantly, studies that have included baseline MRI imaging have found a wide range of pre-existing T2/FLAIR lesion volume, indicating that prior brain injury and gliosis, or white matter disease, are prevalent in this patient population.
In addition to successful use of Sentinel with TAVR, a number of small feasibility studies have shown promise for the use of Sentinel with other endovascular therapies, including LAAO, TEVAR, MitraClip and valve-in-valve procedures.
Safety Outcomes
Given the ease of use of the device and the high procedural success rates, it is not surprising that acute procedural events and 30-day safety events related to the use of Sentinel are rare. Published reports of Sentinel demonstrate consistently low rates of mortality and neurological events, and randomised trials have documented similar major adverse cardiac and cerebrovascular event rates in patients treated with Sentinel (Table 1). In the largest randomised study, SENTINEL, the Sentinel device was easily delivered and was compatible with standard TAVR workflow.13 Total procedure time was increased by approximately 13 min, and fluoroscopy time was increased by 3 min.13 The safety profile of Sentinel is underscored by a >92 % procedural success rate in more than 866 patients across 11 studies, and for multiple endovascular therapies.1,3,5,12,13
Neurological Events
The 30-day rates of overt neurological events, both stroke and transient ischemic attack, were low across all published studies of the Sentinel device. In the CSI-Ulm trial, the largest consecutively enrolled cohort of Sentinel in a commercial setting (n=802), the overall 7-day stroke rate when Sentinel CEP was used was 1.4 %, representing a statistically significant reduction when compared to a cohort of subjects who did not receive CEP at 4.2 % (p=0.03).36 A similar trend was observed in the randomised SENTINEL study (n=363), wherein the periprocedural (≤72 h) stroke rate for Sentinel-protected patients was reduced by 63 %; 3.0 % for patients protected with Sentinel, versus 8.2 % (p=0.05) for unprotected patients.37 When considering the more sensitive endpoint of any worsening of the National Institute of Health Stroke Scale, a study-level meta-analysis by Guistino et al. found a strong trend in favour of TAVR with cerebral embolic protection (M-H Risk Ratio 0.55 [0.27, 1.09], p=0.09) further supporting the results observed in both the CSI-Ulm and Sentinel trials.38 These positive trends in stroke reduction are particularly important when considering the numerous studies that have confirmed the occurrence of stroke post-TAVR.
Mortality
Seven studies have reported 30-day mortality after TAVR with Sentinel, with rates ranging from 0 to 3 %. Within the three randomised trials in which TAVR with Sentinel protection was compared to unprotected TAVR, no significant differences in 30-day mortality were observed between the device treatment and control groups, although deaths in the Sentinel groups were consistently numerically lower (Table 1).
In addition to studies focused on the Sentinel device alone, a recent study-level meta-analysis of all randomised control trials for CEP to date found that, as a class, CEP devices showed a decreased risk of stroke and mortality over unprotected TAVR, which corresponded to an approximately 4.0 % reduction in absolute risk.35
Other Clinical Events
Because the deployment and retrieval of the Sentinel device slightly lengthens the TAVR procedure, there was some early concern about potential increases in acute kidney injury. The low rates of Stage 3 acute kidney injury reported in the early Netherlands experience (2.5 %)1 and SENTINEL Study (0.4 %)13 alleviate these concerns. Low rates of bleeding and vascular complications are similarly reassuring. In the SENTINEL Study, only 1 out of 231 patients (0.4 %) experienced a major vascular complication, a pseudoaneurysm, related to brachial access for the Sentinel device.
Histopathology and Morphometry
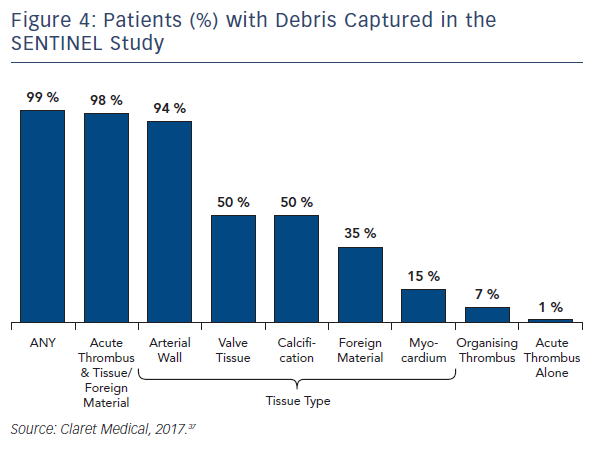
Seven of the published studies, as well as several conference proceedings, have reported histopathological analyses of debris captured by the Sentinel device. The methods for the histopathological analyses were similar across the studies and were performed by the same independent core laboratory. As described previously, regardless of the primary procedure (TAVR, Mitraclip, LAAO, TEVAR), all studies reported near ubiquitous capture of debris removed from the Sentinel device. Rates for debris capture per patient ranged from 75 to 100 % across studies. In order to better understand the prevalence and etiology of the debris captured by the filters, the Sentinel-H Study was conducted. Sentinel-H was a multicentre, all-comers study of 217 patients in Europe. In accordance with other Sentinel studies, debris was captured in 99 % of patients. As with other Sentinel studies, acute thrombus was found most commonly (85 %), and nearly always in conjunction with tissue-derived material, including arterial wall, valve tissue, calcification and myocardium. Organising/organised thrombus and foreign material were also common.39 In addition to the high rates of debris capture (Figures 3 and 4), the SENTINEL Study discovered that 1 in 4 patients had, on average, 25 particles in their filters that were greater than or equal to 0.5 mm in size.
Conclusion
Despite advances in TAVR therapies and techniques, stroke is and will continue to be the Achilles heel of structural heart and endovascular procedures. Indeed, the complexity of discriminating between clinical condition-related strokes and cardiac intervention-related strokes needs further refinement, but all would agree that reducing stroke, regardless of cause, should be a goal. Sentinel is a good step toward that goal. Across multiple studies, Sentinel has demonstrated a strong safety profile and a >92 % procedural success rate. The ease of use of the Sentinel device, combined with minimal disruption to the normal TAVR workflow, make it a viable adjunct therapy that could soon be considered a standard of care for TAVR, and possibly for other cardiac and vascular interventions. Studies to date using Sentinel have been performed in high-risk surgical patients who have an elevated ‘background noise’ of medical history. However, Sentinel has now been cleared by the US FDA to be used in all TAVR patients regardless of surgical risk at the same time that TAVR expands to treat moderateand low-risk patients and potentially asymptomatic aortic stenosis patients. New repositionable TAVR devices facilitate more precise valve implantation but may cause higher embolic burden, which will further emphasise the role of cerebral embolic protection.