International guidelines recommend primary prevention ICDs (PPICDs) in patients with symptomatic heart failure and a left ventricular ejection fraction (LVEF) <35% after a minimum 3 months of optimal medical therapy (OMT).1 While LVEF reassessment is advised 6–12 weeks after acute MI (AMI) if acutely <40% to permit potential recovery of LVEF after revascularisation given the phenomena of myocardial stunning.
However, less than 20% of sudden cardiac deaths (SCD) occur in patients with an LVEF of <35%.2 Furthermore, prevalence of SCD is highest in the first 6 months post-AMI, with an estimated 51% of these attributable to ventricular arrhythmias (VA) suggesting strict guideline compliance may risk a delay for truly eligible patients receiving PPICD resulting in potentially avoidable SCD secondary to VA.3
Case Report
A 73-year-old woman presented with progressive exertional dyspnoea and chest pain. Her medical history was significant for hypertension and chronic kidney disease and she was a former smoker. More recently, she had developed orthopnoea and paroxysmal nocturnal dyspnoea prompting her to seek medical attention.
Clinical examination findings were consistent with pulmonary oedema. An ECG demonstrated sinus rhythm with left bundle branch block. Admission blood tests showed a troponin T (TnT) of 46 ng/l (ref <14) and N-terminal pro-B naturetic peptide (NT-proBNP) of 1,828 ng/l (ref <125 ng/ml). Following initial treatment with loop diuretics, she was stabilised with optimal medical therapy (OMT).
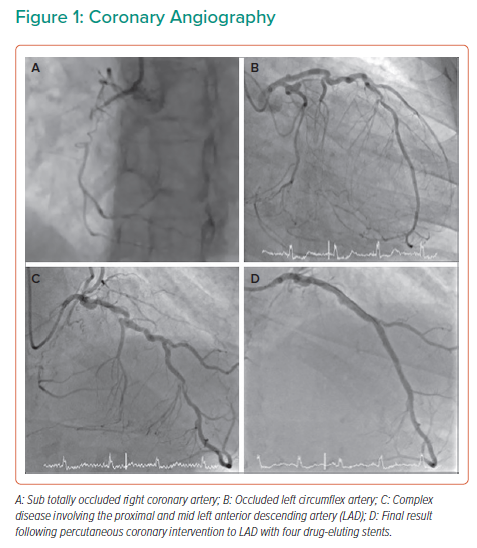
Transthoracic echocardiography demonstrated an LVEF of 24%. Coronary angiography showed severe coronary disease with sub-totally occluded right coronary artery (RCA) (Figure 1A), occluded left circumflex (LCx) (Figure 1B) and severe calcific stenosis of proximal left anterior descending artery (LAD) extending into left main stem (LMS) (Figure 1C).
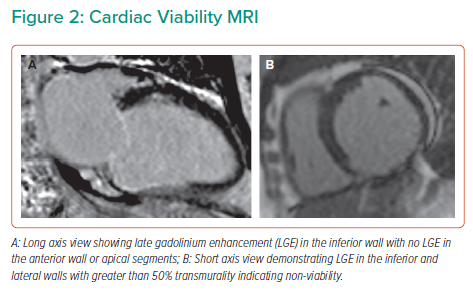
Viability assessment with cardiac MRI (CMRI) was undertaken to inform potential revascularisation, this confirmed severe systolic dysfunction with an LVEF 31% and non-viable RCA and LCx territories (Figure 2). The LAD territory remained viable with anterior wall dysfunction suggesting there would be a potential benefit from revascularisation.
Following heart team discussion, the patient underwent intravascular ultrasound-guided percutaneous coronary intervention (PCI) to LAD/LMS with four drug-eluting stents placed in the absence of mechanical circulatory support (Figure 1D). Asymptomatic loss of septal vessels was noted. Following observation for 48 hours post-PCI, the patient was discharged home on bisoprolol 2.5 mg, ramipril 5 mg, eplerenone 25 mg, aspirin 75 mg and clopidogrel 75 mg. Implantation of a PPICD was considered but ultimately deferred following a multidisciplinary team discussion as per guideline recommendations, pending LVEF reassessment 6 weeks post-revascularisation and initiation of OMT.
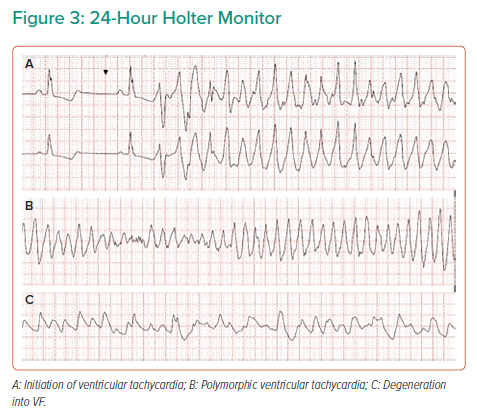
During a cardiac rehabilitation review 3 weeks post-discharge, the patient reported an episode of palpitations and dyspnoea, which had woken her up. A 24-hour ambulatory ECG monitor was fitted and the patient allowed home, but the patient died that night. Subsequent holter interrogation demonstrated sustained ventricular tachycardia (VT) (Figure 3A and B) degenerating into ventricular fibrillation (Figure 3C) leading to SCD.
Discussion
The efficacy of PPICD for ischaemic cardiomyopathy (ICM) was established in two trials. In MADIT II LVEF <30% was used for inclusion and an absolute risk reduction of 6% at 2-year follow-up was found, while SCD-HeFT using LVEF <35% as a cut-off found an absolute risk reduction of 7% after 5 years.4,5 Based on these studies, 17–25 ICDs would be required to save one life suggesting that, while PPICDs are an effective intervention, the use of LVEF in isolation is not the most sensitive method to identify the subset of patients who will receive the most benefit from their use.
The VALIANT registry of 14,609 patients found the rate of SCD at 30 days post-AMI to be 1.4% per month, reducing over time to 0.14% per month at 2 years.6 Of note, if the LVEF was <30%, SCD risk increased to 2.3% in the first month, furthermore 83% of mortality was within 30 days of discharge. Despite this, guidelines advocate waiting 6 weeks after AMI before considering PPICD, based on two randomised control trials.1
DINAMIT enrolled patients 6–40 days post-AMI with LVEF <35% and autonomic dysfunction while IRIS enrolled patients 5–31 days post-AMI with LVEF <40% and either elevated resting heart rate or non-sustained ventricular tachycardia.7,8 Both trials failed to show a benefit in all-cause mortality with the use of a PPICD. While a reduction in SCD was seen, this was offset by an increase in non-SCD. Mechanistically, this could be because 50% of SCD are due to non-arrhythmic causes, such as myocardial rupture and recurrent AMI, and would not be affected by PPICD.3 Alternatively, perhaps LVEF alone has poor specificity for determining arrhythmogenic potential. The trials were performed prior to the routine use of primary PCI and have small sample sizes, which are potential confounders.
More recently, patients with ST-segment elevation AMI and EF <40% were randomised within 4 days of PPICD if VT was inducible in an electrophysiological study (EPS).9 In patients with LVEF <40%, death or VA burden were not different to patients with EF >40% if they had a negative EPS for inducible VT. This again highlights the imprecise nature of using LVEF alone in predicting arrhythmic SCD but suggests EPS may be better at stratifying VA risk compared with autonomic dysfunction used in previous studies.
Performing an EPS in all patients with reduced LVEF following AMI would not be practical and therefore CMRI offers an attractive non-invasive alternative. A meta-analysis of 2,850 patients found the presence of scar, as detected by late gadolinium enhancement (LGE) on CMRI, to be associated with arrhythmic events.10 The annualised arrhythmia event rate in patients with LGE was 8.6% compared with 1.7% in those without LGE. In patients with LVEF <30% and LGE, 25.8% had an arrhythmic event in contrast to 3.1% in those without LGE. These patients can be further risk stratified by evaluating scar mass, transmurality and gray zone mass, in particular.11 The gray zone describes heterogeneous tissue with mixed fibrosis and viable myocytes in the border zone between infarcted and normal myocardium. This substrate permits re-entrant circuits to develop and may be more arrhythmogenic than fully infarcted tissue. Gray zone mass has also been correlated with arrhythmia risk and inducibility of VT on EPS.2,12 In this case, the presence of transmural scar in the inferior and inferolateral wall with a further >50% scar in the lateral wall suggested the patient was at high risk for VA, and using such characteristics could help refine selection for PPICD by better risk stratifying patients as compared to using EF in isolation.
The impact of revascularisation on scar characteristics is yet to be well described. A small series of three patients undergoing chronic total occlusion PCI found a reduction in the area of border zone scar at 6 months post-PCI.13 Whether this translates to a reduction in VA risk or improved outcomes requires further study. Additionally, in the present case the loss of septal vessels during PCI may have provided a nidus for triggering VA potentially by creating a new scar.
A number of studies have consistently shown that LVEF is less likely to improve when impairment is due to ICM.14 Surgical revascularisation with coronary artery bypass grafting (CABG) in ICM does improve outcome, but only after a decade, as shown in the STICH study.15 However, further analysis found CABG did not increase the chances of LVEF improvement of >10% as compared with OMT.16 Improvement in LVEF >10% and CABG both independently improved survival, leading the authors to conclude CABG improves survival by mechanisms other than LVEF recovery. The recent REVIVED study found no benefit to revascularisation with PCI compared to OMT, moreover PCI did not affect LVEF above the improvements seen with OMT alone.17
A registry of 2,540 PPICD implants following guideline recommendations found the probability of appropriate device therapy at 3 years was 24% while 12% received inappropriate therapy.18 Moreover, at 12 months there was no difference in the probability of receiving an appropriate or inappropriate shock (6.1% versus 5%, p=0.06). Inappropriate shocks have been associated with increased mortality, with the risk increasing with each inappropriate shock experienced, reinforcing the importance of patient selection for PPICD.19
A compromise in scenarios such as the present case study may be to use a wearable cardioverter defibrillator (WCD) while awaiting reassessment of LVEF. WCD can successfully treat VA while also allowing inappropriate shocks to be aborted via a patient response button in up to 95% of cases where incorrect VA detection has occurred.20
Finally, medical therapy must always be optimised in such cases to reduce the risk of VA and SCD irrespective of whether a PPICD is implanted. The present case demonstrates initiation of VT following a ventricular ectopic causing R-on-T phenomenon and dose titration of ß-blocker may have reduced the risk of ectopy. Moreover, ß-blockers have been shown to reduce the risk of SCD by 31% in heart failure.21
Conclusion
We propose that current guidelines for PPICD lack sensitivity and specificity for patient selection where LVEF is the sole determinant. As in this case report, the arrhythmic risk could have been better predicted to prompt PPICD implantation during the index admission without waiting 6 weeks when there is significant myocardial scar and the benefits of revascularisation in ICM are unclear. We advocate a more personalised approach to risk stratification of such patients.
Clinical Perspective
- Current guideline recommendations result in many patients receiving a primary prevention implantable cardioverter defibrillator (PPICD) from which they may never receive therapy or receive inappropriate therapies and conversely some patients in whom sudden cardiac death could have been aborted will not receive an ICD.
- Left ventricular ejection fraction has poor sensitivity and specificity for identifying patients at high risk of sudden cardiac death from ventricular arrhythmia in ischaemic cardiomyopathy.
- Scar characteristics on cardiac MRI may improve the identification of patients who would most benefit from a PPICD.
- The role of revascularisation in ischaemic cardiomyopathy to improve ventricular function and reduce arrhythmic risk remains unclear.