Transcatheter aortic valve implantation (TAVI) has become a commonly used and minimally invasive approach for patients with severe aortic stenosis (AS).1,2 In the latest guidelines from the 2017 European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) for the management of valvular heart disease, the following clinical characteristics favour TAVI: Society of Thoracic Surgeons (STS)/EuroSCORE II ≥4 % (logistic EuroSCORE I ≥10 %); presence of severe comorbidity; age ≥75 years; and presence of frailty or restricted mobility and conditions that may affect the rehabilitation process after the procedure.3 Accumulated data from randomised controlled trials and large registries of elderly patients show that TAVI is superior to medical therapy in terms of mortality in extreme-risk patients4, non-inferior or superior to surgery in high-risk patients,5–8 and non-inferior to surgery and even superior when transfemoral access is possible in intermediate-risk patients.9–12 The second-generation self-expanding transcatheter heart valve (THV) also showed substantial efficacy with a low rate of vascular complications compared with older devices.13 It is expected that more patients will be candidates for TAVI in the future. Although this technique has reached relative maturity, further optimisation of patient selection and device implantation is essential to improve prognosis.
Pre-procedural assessment of imaging techniques, specifically, multi-detector computed tomography (MDCT), has been recommended for TAVI optimisation.14
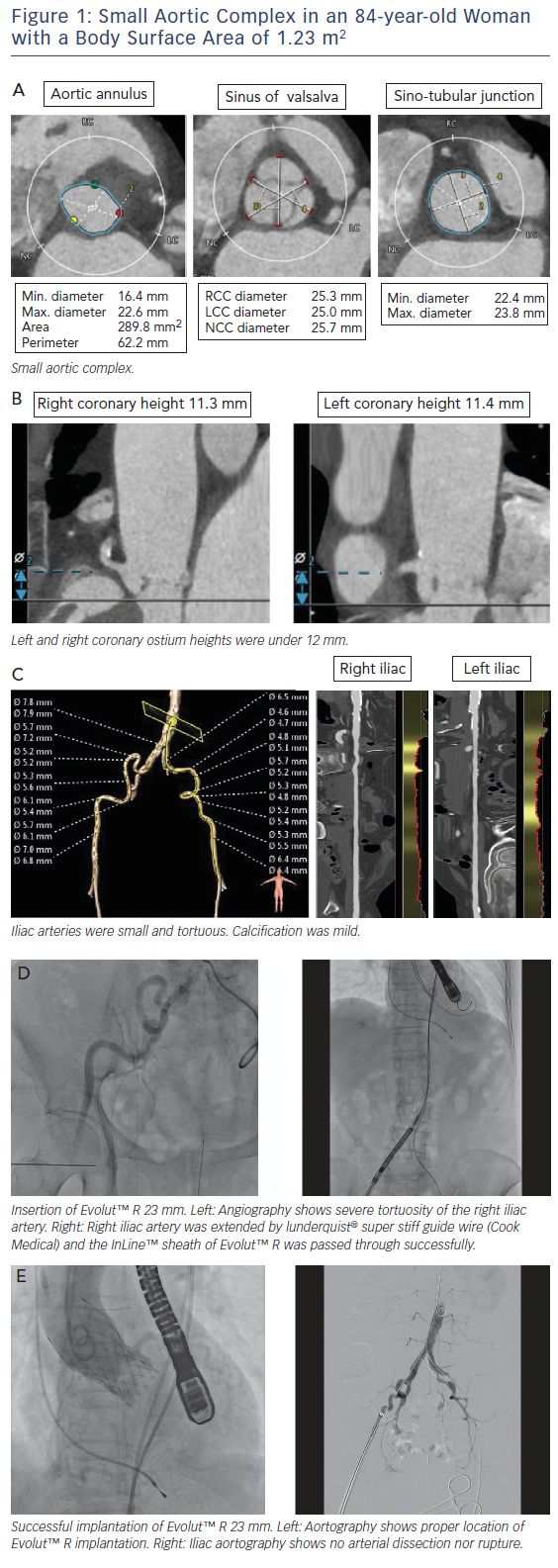
Asian populations have smaller body size (BSA=1.41±0.15 m2), aortic annulus size, and vascular access size than European populations (BSA=1.72±0.18 m2);15 this is a potential risk for annulus rupture, residual aortic valve stenosis, or vascular complications.16,17 Women with severe AS had smaller aortic root dimensions, even after correcting for their smaller body size and height, reflecting a sex-specific difference.18 This review includes a discussion of issues related to a small aortic complex and small access arteries. These issues are illustrated in a short case study provided in Figure 1.
Small Aortic Annulus
MDCT is useful to decide on the TAVI strategy in relation to access site, valve type and valve size. The incidence of annulus rupture or perforation is as low as 0–1.1 %; however, this is a catastrophic complication associated with a high risk of death.19,20 Aggressive device oversizing and large calcifications in the epicardial fat area of the annulus/left ventricular outflow tract have been reported as risk factors for annulus rupture.19,21,22 A smaller annulus calculated by MDCT (e.g. annulus area <300 cm2) may increase the risk of annulus rupture due to relative valve oversizing.21 A previous report showed a trend towards higher incidence of annulus rupture in patients with a small body size (BSA <1.75 m2) compared with patients with a larger body size (BSA ≥1.75 m2) (2.3 % versus 0.5 %; p=0.11).23
Prosthesis–Patient Mismatch
Prosthesis–patient mismatch (PPM), defined by an indexed effective orifice area <0.85 cm2/m2 using echocardiography, is a major concern after aortic valve replacement. The risk factors for PPM are small annulus diameter and larger BSA.24 PPM is common (20–70 %) after surgical aortic valve replacement (SAVR), and has a negative impact on short- and long-term outcomes.25 Head et al. reported that moderate and severe PPM are associated with a 1.2- and 1.8-fold increase in the risk of all-cause mortality, respectively.26 Some studies have reported that PPM is associated with less regression of left ventricular hypertrophy, less improvement in patient functional status and increased mortality after TAVI,27,28 whereas other studies found no significant impact of PPM on outcomes.29 Pibarot et al. reported that PPM is more frequent and often more severe after SAVR than after TAVI, and that TAVI may be preferable to SAVR in patients with a small aortic annulus who are susceptible to PPM, to avoid the adverse impact on left ventricular mass regression and survival.30 Distention of the aortic annulus due to systematic oversizing and the absence of a sewing ring may have been potential mechanisms accounting for the superior haemodynamic profile associated with TAVI compared with standard surgical valves.31
Acute Coronary Obstruction
The coronary height from the aortic annulus plane to the coronary ostium is also a matter of great concern for TAVI in patients with a small body size. Acute coronary obstruction (ACO) is thought to be caused by native-valve leaflet involvement in the coronary ostium among patients undergoing TAVI.32 A previous study showed the distance between the left coronary ostium and the aortic annulus plane was shorter in the small-body group.23 Rebeiro et al. reported on data from a multicentre registry that showed that both coronary height <12 mm and sinus of Valsalva <30 mm were powerful predictors of ACO.33 Yamamoto et al. reported females were more prevalent in the coronary protection (CP) group than in the non-CP group (89.4 % versus 67.3 %), resulting in a smaller body height and body surface area in the CP group in the Japanese population. They also reported ACO occurred in 70 % of patients with bulky calcification, 50 % of
patients with leaflet length exceeding target coronary height, and 20 % of patients with flow limitation during valvuloplasty with simultaneous aortic root injection. Although the complication of ACO was difficult to predict, a preparatory CP strategy, such as guidewire insertion with or without a therapeutic balloon, is safe and feasible for the management of ACO during TAVI.34
Small Femoral Artery
Asian populations, including Japanese, have a smaller femoral artery diameter compared with Europeans.15 Vascular complications are relatively frequent and serious in transfemoral TAVI, and previous reports have shown that vascular complications are associated with significantly increased patient morbidity and mortality.35,36 The ratio of the sheath outer diameter (in millimetres) to the minimal femoral artery diameter (in millimetres) >1.05 is a predictor of vascular complications.35 In previous reports, newer TAVI technology with a lower-profile sheath system, SAPIEN 3 (Edwards Lifesciences), reduced bleeding and vascular complications by reducing sheath size using 14Fr or 16Fr Edwards eSheath (minimum artery diameter 5.5 mm). Arai et al. reported that SAPIEN 3 had a lower incidence of vascular complications (3 %) than those of SAPIEN XT (Edwards Lifesciences) implantation (12 %) via the femoral artery.37 The second-generation self-expanding transcatheter heart valve (THV) EvolutTM R (Medtronic) also showed substantial efficacy, with a low rate of vascular complications by reducing sheath size using 14Fr-equivalent system with InLineTM (minimum artery diameter 5.0 mm), which is adaptable to smaller access arteries compared with SAPIEN 3.13 Subclavian access with EvolutTM R was not significantly different from transfemoral access and may represent the safest non-femoral access route for TAVI.38
Summary
Small body size is more common in Asians and women and is associated with small aortic annulus size, low coronary height and smaller femoral artery size. Patients with small body size have a potential risk for annulus rupture, ACO, PPM and vascular complications during a TAVI procedure. The surgical approach to a patient with a small annulus has a risk of PPM, and TAVI is superior to SAVR, especially in terms of PPM, so we should consider TAVI for patients with a small annulus. Selection of THV in a patient with a small annulus is the next consideration. The area of the SAPIEN 3 THV is smaller than that of the SAPIEN XT when opened with the same nominal pressure and same size valve due to an outer skirt attachment. Therefore, the S3 20 mm THV may have potential risk of PPM.39 The EvolutTM R is designed for a supra-annular position to increase the effective orifice area, and may be suitable in patients with a small annulus. Pre-procedural planning with CT scan is quite important to determine the selection of THV and avoid complications.
Conclusion
Small body size is a predictor of a challenging TAVI procedure. TAVI with new generation devices is superior to SAVR and has a lower incidence of PPM. A self-expanding supra-annular THV would be favourable in a patient with a small annulus.