Transcatheter aortic valve replacement (TAVR) with either the balloonexpandable Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, California), or the self-expandable CoreValve prosthesis (Medtronic, Minneapolis, Minnesota) has become the established therapeutic modality for severe aortic valve stenosis in patients who are not deemed suitable for surgical intervention due to excessively high operative risk. In the pivotal Placement of Aortic Transcatheter Valves (PARTNER) randomized trial, TAVR with the Edwards SAPIEN valve prosthesis, compared with conventional therapy, decreased the rate of death at 1 year, reduced cardiac symptoms, and was associated with improvement in valve function.1 Medium-2 and longer-term3 follow-up studies of the PARTNER trial patient cohort demonstrated sustained benefit of TAVR over standard therapy in terms of mortality, functional status, and hemodynamic performance of the aortic valve. In addition, in high-risk patients with severe aortic stenosis (AS), transcatheter aortic valve implantation and surgical replacement of the aortic valve were associated with similar rates of survival at 1 year4 and 2 years.5
Native aortic valve regurgitation (NAVR), defined as primary aortic regurgitation not associated with AS or failed surgical or TAVR, is still considered a relative contraindication for transcatheter aortic valve therapies. In fact, in the PARTNER trial, patients with severe (grade ≥3) aortic incompetence were excluded from the study, because of the absence of annular or leaflet calcification required for secure anchoring of the transcatheter heart valve and the associated risk for valve dislocation.1 In addition, severe aortic regurgitation often is accompanied by aortic root or ascending aorta dilatation, the treatment of which mandates operative intervention.
In recent years, TAVR has been sporadically used to treat NAVR in surgically inoperable patients. Initial reports described transcatheter deployment of the Medtronic CoreValve prosthesis or the Edwards SAPIEN XT valve to treat NAVR on a compassionate basis,6–9 and in special settings such as left ventricular (LV) assist device,10,11 or heart transplantation.12 The initial experience has been comprehensively reviewed by Roy et al.13
More recently, TAVR for isolated aortic regurgitation has been performed with newer-generation valves, such as the JenaValve (JenaValve Technology, Munich, Germany), the ACURATE TA valve (Symetis, Ecublens, Switzerland), and the Medtronic Engager valve (Medtronic 3F Therapeutics, Inc., Santa Ana, California, US).14 In addition, new ancillary devices such as the Helio dock have emerged that are specifically designed to stabilize the annulus–root complex, and have been trialled in conjunction with older-generation transcatheter aortic valve prostheses.15
Aortic Regurgitation and Indications for Valve Replacement
NAVR arises from failure of the aortic valve leaflets to appose during ventricular diastole, a process that may occur because of leaflet damage or distortion or from dilatation of the ascending root. Whereas a fibro-calcific degenerative process accounts for the large majority of cases of AS, the aetiologic spectrum of aortic incompetence is much broader. Globally, rheumatic heart disease accounts for a large burden of isolated aortic incompetence. In the developed world, degenerative and congenital conditions, such as bicuspid aortic valve, are more common causes of isolated aortic regurgitation.16 Enlargement of the aortic root and ascending aorta can occur concurrently and are frequently interdependent. The prevalence estimates of NAVR vary widely depending of the definition of aortic regurgitation used and the characteristics of the population: 2–30 %.16 The prevalence of significant NAVR in the general population is estimated at 1 %.16
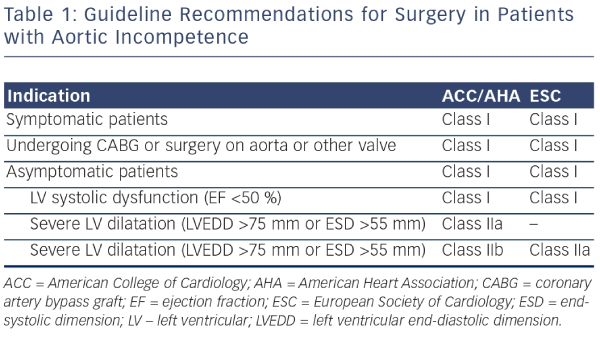
Patients with mild aortic regurgitation rarely develop significant symptoms or LV dysfunction: even severe aortic regurgitation can remain indolent for a prolonged period of time. Symptoms may develop, however, once the imbalance between diastolic LV distension, myocardial muscle mass, and systolic wall tension leads to progressive LV dilatation and systolic dysfunction. Prognosis in patients with severe aortic regurgitation is associated with both the presence of symptoms and LV dysfunction. In a study of 246 patients with moderate of severe aortic regurgitation, 10-year mortality was closely associated with symptoms: those in New York Heart Association (NYHA) class II–IV had an annual mortality of 25 % compared with the 3 % mortality seen in asymptomatic patients.17 In addition, patients with LV dysfunction had a threefold greater mortality than those with preserved function.17 A long-term postoperative study has demonstrated improved survival when patients undergo early AVR after onset of mild symptoms, mild LV dysfunction (ejection fraction [EF] 45 % to 50 %), or end-systolic dimension 50 to 55 mm rather than waiting for more severe symptoms or more severe LV dysfunction to develop.18 Accordingly, the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines recommend aortic valve replacement when symptoms supervene, or once LV dilatation (LV end-systolic diameter >55 mm) or LV dysfunction (LVEF <50 %) develop (see Table 1).19
TAVR for Native Aortic Valve Regurgitation— Experience with the First-generation Transcatheter Heart Valves
Initial attempts at catheter-based treatment of NAVR in patients deemed surgically inoperable used first-generation transcatheter heart valves, such as the CoreValve and the Edwards SAPIEN valve. The CoreValve has hitherto been the preferred valve because of its technical and design characteristics. In particular, self-expandable valves, such as the CoreValve, offer high and permanent recoil forces that are thought to provide better stability in the noncalcified native aortic valve apparatus compared with the balloon-expandable transcatheter valves. Though neither valve was designed to treat this condition, successful cases have been reported.
Edwards SAPIEN
The Edwards SAPIEN heart valve system, which consists of a tri-leaflet bovine pericardial valve and a balloon-expandable, stainless steel support frame, has been used infrequently in the treatment of aortic regurgitation. One report describes the trans-apical deployment of a 29 mm valve via mini-thoracotomy and under cardiopulmonary bypass for a patient who developed severe aortic incompetence despite a structurally normal valve following LV assist device implantation.10 The procedure required substantial over-sizing (29 mm valve for a 21 mm annulus that would normally require a 23 mm valve). No residual aortic incompetence was noted, but no follow-up data were provided. Another case report documented procedural failure, with deployment of a 26 mm Edwards SAPIEN valve prosthesis in a patient with severe AR and an aortic annulus diameter of 26 mm, followed by valve dislocation soon after deployment and embolization into the left ventricle.20 A 29 mm CoreValve was then deployed successfully after retrieval of the embolized Edwards SAPIEN valve.
CoreValve
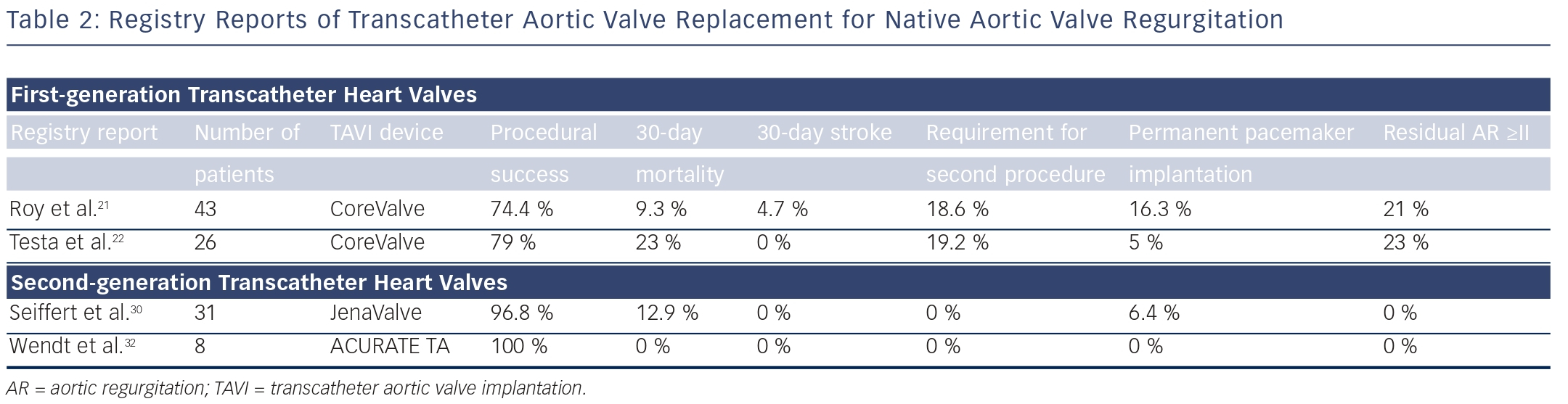
The CoreValve prosthesis consists of a tri-leaflet biological valve sewn into a self-expanding nitinol frame. An international registry study demonstrated the feasibility and potential procedural difficulties when using TAVI for severe AR21 (see Table 2). The study described 43 patients who underwent TAVR for NAVR with the CoreValve between 2011 and 2012 at 14 centers worldwide. All patients had pure severe AR and were deemed surgically inoperable with mean logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE), 26.9±17.9 %; and mean STS (Society of Thoracic Surgeons) score, 10.2±5.3 %. Although procedural success was high, with successful implantation of a TAVR in 42 out of 43 (97.7 %) patients (one conversion to open heart surgery), eight patients (18.6 %) required a second valve during the index procedure for residual aortic regurgitation. Post-procedure aortic regurgitation grade II or higher was present in nine patients (21 %), meaning that the Valve Academic Research Consortium (VARC)-defined procedure success was reduced to 74.4 %.21 At 30 days, the major stroke incidence was 4.7 %, and the allcause mortality rate was 9.3 %. At 12 months, the all-cause mortality was 21.4 % (six out of 28 patients). Also of interest was the finding that the need for a second valve was limited to patients without aortic valve calcification. This study demonstrated the feasibility of this technique and highlighted the potential procedural difficulties in treating NAVR with TAVR.
In a large CoreValve registry from Italy, 1,557 consecutive patients undergoing TAVR, of whom 26 (1.6 %) presented with AR, were prospectively followed and baseline clinical characteristics and clinical outcomes compared22 (see Table 2). Patients with AR were significantly younger (mean age 73±10 versus 82±6), more frequently suffered from advanced heart failure symptoms (NYHA Class III/IV 95 % versus 73 %), and had a higher incidence of severe pulmonary hypertension (SPAP >60 mmHg, 31 % versus 10 %) compared with patients with AS. Logistic EuroSCORE and STS scores were similar between the two groups and VARC-defined device success was lower in the AR group (79 % versus 96 %). At 1 month, patients treated for AR had a higher overall mortality (23 % versus 5.9 %, odds ratio [OR] 4.22) and cardiac mortality (15.3 % versus 4 %, OR 4.01). At 12 months, overall and cardiac mortality remained higher for patients who underwent TAVR for AR compared with AS (31 % versus 19 %, hazard ratio [HR] 2.1, and 19.2 % versus 6 %, HR 3.1, respectively).22
TAVR for Native Aortic Valve Regurgitation— Experience with the Newer-generation Transcatheter Heart Valves
A number of newer-generation transcatheter heart valves have been developed in recent years23 (see Table 2). Four devices that may be particularly suited for catheter-based treatment of aortic regurgitation are the JenaValve, ACURATE TA valve, the Medtronic Engager valve, and the J-valve. In fact, the lack of calcification of the valvular apparatus confers greater stability to some newer-generation valves, and hence allows these devices to work more effectively. These valves share several characteristics. They are deployed primarily transapically; they are self-seating; they are anatomically oriented into the commissures; and they can be deployed in animal models in the orthotopic position because of their lower radial force compared with other TAVR devices.
The JenaValve
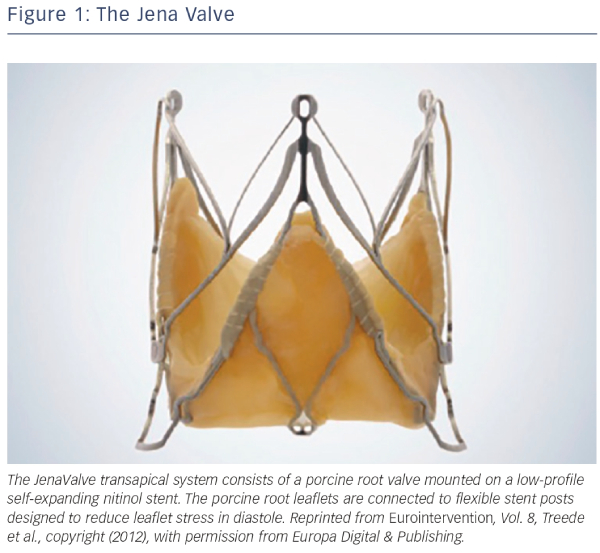
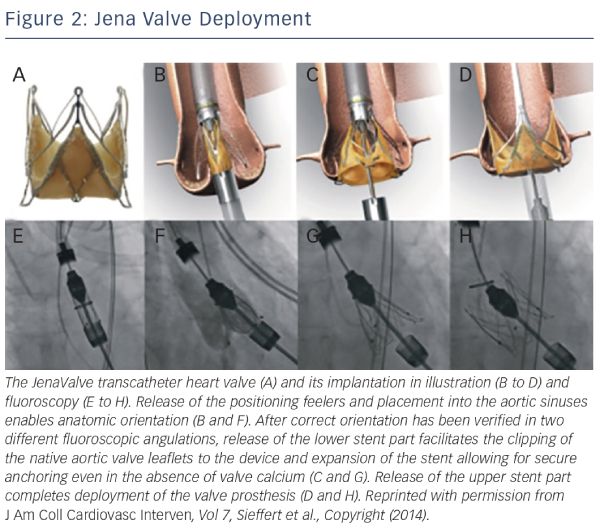
The JenaValve transapical system consists of a porcine root valve mounted on a low-profile self-expanding nitinol stent24 (see Figure 1). The porcine root leaflets are connected to flexible stent posts designed to reduce leaflet stress in diastole. In contrast to devices expanding within the aortic annulus, the JenaValve relies on an active clip fixation of the native aortic valve leaflets, thereby reducing the radial force applied on surrounding cardiac and aortic structures24 (see Figure 2). This design also prevents coronary compromise by the native leaflets or stent struts, and does not interfere with future coronary intervention. Because the valve deploys in an anatomically aligned position, rapid pacing and balloon inflation is not required during implantation.
Several initial reports demonstrated feasibility and successful treatment of aortic regurgitation using the JenaValve.25–30 In the largest series of 31 patients, transapical TAVR with a JenaValve for the treatment of NAVR was performed in 31 patients in nine German centers.30 Average age at time of procedure was 74 years, and all patients were deemed at high risk for cardiac surgery, with an average logistic EuroSCORE of 23.6 %. Procedural success was achieved in 30 out of 31 cases, with one patient requiring a second valve implantation due to valve dislocation. Two patients underwent either surgical or further transcatheter valvular re-interventions because of infective endocarditis or for increasing paravalvular regurgitation. All-cause mortality was 12.9 % and 19.3 % at 30 days and 6 months, respectively. Cerebrovascular events did not occur during follow-up, and a significant improvement in NYHA class was observed and persisted up to 6 months. Post-procedural aortic regurgitation was none/trace in 28 of 31 and mild in three of 31 patients. At present, the JenaValve is the only transcatheter valve that has been CE-marked for treatment of both AS and aortic regurgitation.
ACURATE TA Valve
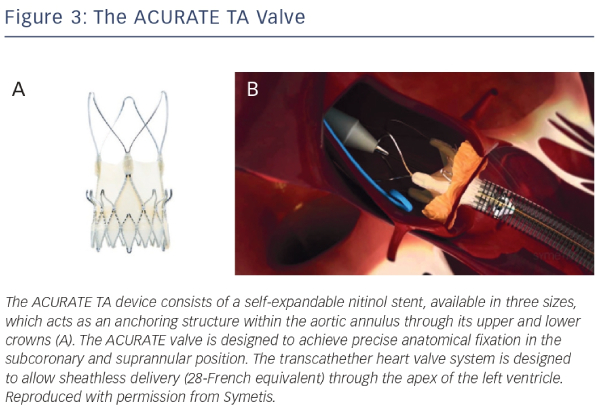
The ACURATE TA device (Symetis SA Ecublens, Switzerland) consists of a self-expandable nitinol stent, available in three sizes, which acts as an anchoring structure within the aortic annulus through its upper and lower crowns23,31,32 (see Figure 3). The ACURATE valve is designed to achieve precise anatomical fixation in the subcoronary and suprannular position. A three-leaflet porcine valve is fixed to the lower part of the nitinol stent. A double polyethylene skirt covers the inner and outer surface of the stent body, thus reinforcing the porcine valve and avoiding direct contact between the biological tissue and the metal stent struts. The transcathether heart valve system is designed to allow sheathless delivery (28-French equivalent) through the apex of the left ventricle. Intra-procedural balloon valvuloplasty is generally not required for deployment, and implantation is carried out without rapid pacing.
Wendt et al. describe procedural characteristics and clinical outcomes in a series of six patients with severe native aortic valve incompetence and two patients with aortic regurgitation secondary to failed aortic root repair, all of whom were deemed surgically inoperable in view of mean logistic EuroSCORE and STS scores of 34 % and 7.3 %, respectively.32 Procedural success was 100 % and no complications satisfying the VARC-2 criteria occurred in the peri-procedural period. Residual aortic incompetence post-procedure, as documented by transesophageal echocardiography, was at most grade I at three and six months’ follow-up. At 12 months’ follow-up, all-cause mortality, cardiovascular mortality, and cerebrovascular event rates were zero.32 Currently the ACURATE transcatheter valve is only available for transapical implantation, but a transfemoral version, the ACURATE TF, is undergoing clinical trials. Given the encouraging initial results, the ACURATE TA transcatheter heart valve might become more widely tested in the treatment of NAVR.
Medtronic Engager
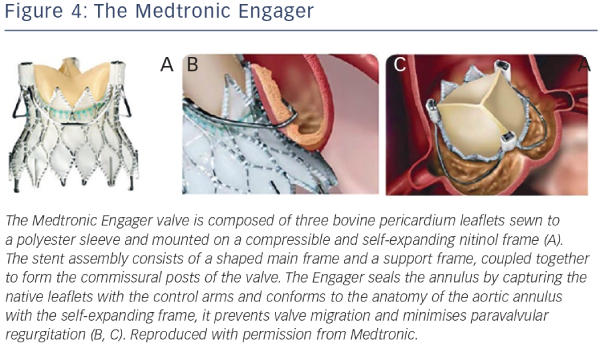
The Medtronic Engager aortic valve system (Medtronic 3F Therapeutics, Inc., Santa Ana, California, US) is a second-generation TAVR bioprosthesis combined with a delivery system designed for over-the-wire transapical implantation of the valve. The valve is composed of three bovine pericardium leaflets sewn to a polyester sleeve and mounted on a compressible and self-expanding nitinol frame23,33 (see Figure 4). The stent assembly consists of a shaped main frame and a support frame, coupled together to form the commissural posts of the valve. The Medtronic Engager valve obtained CE (Conformité Européenne) mark in 2013, after the pivotal multicentre trial demonstrated feasibility and acceptable safety profile in 125 patients with inoperable AS.23
Because the Engager seals the annulus by capturing the native leaflets with the control arms and conforms to the anatomy of the aortic annulus with the self-expanding frame, it prevents valve migration and minimizes paravalvular regurgitation (see Figure 4). Although primarily intended for the treatment of severe AS, it has the potential to develop a role in the treatment of aortic regurgitation, given its unique technical design. Indeed, the Engager has been successfully used to treat noncalcific, severe aortic insufficiency in an inoperable patient with an excellent final echocardiographic result.34 However, the patient required permanent pacemaker implantation, a complication that occurred in a significant number of patients (28 %) recruited into the initial multicentre trial previously alluded to.33
J-valve
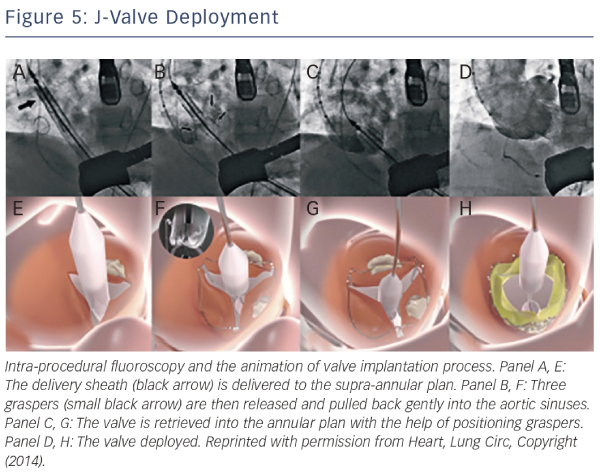
The J-valve system (Jie-cheng Medical Technology, Suzhou, China) is a newly developed second-generation transcatheter heart valve designed for anterograde transapical implantation characterised by three U-shaped graspers that facilitate anatomically correct valve implantation and provide axial as well as radial fixation by embracing the native valve leaflets35 (see Figure 5). The first-inhuman implantation for a high-risk patient with severe NAVR was successful and excellent valve function was demonstrated at followup35 (see Figure 5).
Ancillary Devices
Helio Dock
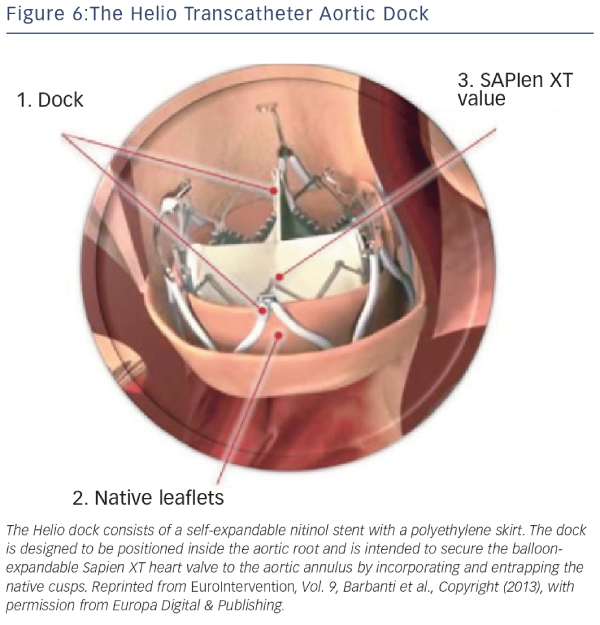
The Helio transcatheter dock (Edwards Lifesciences, Irvine, California, US) is an ancillary device that is intended to confer greater annular stability to the Edwards SAPIEN XT valve.15,36 The dock consists of a self-expandable nitinol stent with a polyethylene skirt (see Figure 6). The dock is designed to be positioned inside the aortic root and is intended to secure the balloon-expandable SAPIEN XT heart valve to the aortic annulus by incorporating and entrapping the native cusps. Following the first in-human successful report, a feasibility study of the procedure was demonstrated in a small series of four patients with severe primary AR deemed inoperable.37–39 Although initial results were encouraging, the dock technique has not gained traction, presumably due to the complexity of the technique and imminent availability of next-generation valves. At present, the manufacturer has discontinued production of the Helio dock.
Technical Considerations
Patient Selection
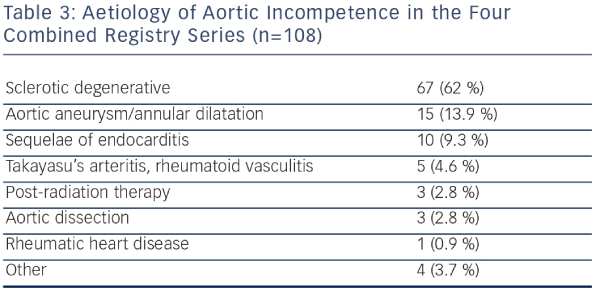
The use of TAVR for NAVR should still be limited to inoperable patients or those with prohibitive surgical risk due to age, frailty, or co-morbid conditions, as the clinical evidence accumulated thus far is not sufficient to establish noninferiority of TAVR compared with surgical treatment. From the Italian CoreValve registry, compared with inoperable patients with AS, patients who underwent TAVR for NAVR were younger, had more advanced NYHA class symptoms despite standard medical therapy, more frequently had SPAP, and had larger LV end-systolic as well as end-diastolic volumes. In the combined four largest series of TAVR for AR (a total of 108 patients), the predominant etiology of aortic incompetence was sclerotic-degenerative in 62 % of patients, followed by aortic aneurysm or dilatation in 13.9 % (see Table 3), with radiation therapy, infective endocarditis, rheumatic heart disease, vasculitis, and connective tissue diseases accounting for the remainder. The significant number of patients with aortic aneurysms is surprising, given that aortic regurgitation with concomitant aortopathy would normally mandate consideration of thoracic aortic surgical replacement with or without valve surgery.
Acute aortic incompetence is generally attributable to dissection of the ascending aorta or infective endocarditis, and is not likely to be amenable to satisfactory transcatheter treatment.
Diagnostic Work-up
Multimodality imaging, consisting of transthoracic and transesophageal echocardiography, and multidetector computed tomography imaging has been used increasingly in preparation for TAVR with the firstgeneration transcatheter valves, and is now accepted as standard practice. Contrast-enhanced multislice computed tomography is particularly useful for assessing valve and annular anatomy and aortic root morphology and should now be considered standard practice for planning TAVR procedures where available. Effective aortic annulus diameters, areas, and perimeters are often derived from multiplanar reconstruction of computed tomography data and/or from the midesophageal long-axis view in transesophageal echocardiography. In cases of NAVR, where precise valve dimensions are essential to prevent valve dislocation, multimodality imaging is essential.
Procedural Details
The newer-generation transcatheter valves are designed for implantation predominantly via the transapical route in the hybrid cardiac catheterization laboratory. Rapid or burst pacing is generally not required, as is balloon inflation and dilatation. Compared with TAVR for AS, TAVR for aortic incompetence has proved to be more technically challenging as reflected by the higher rate of valve-in-valve procedures required, and the lower rate of procedural success.
The absence of annular or cusp calcification translates into a lack of fluoroscopic markings that may make valve positioning more arduous compared to patients with calcific AS. The use of two pigtail catheters, the tips of which are positioned in the noncoronary and left coronary cusps may facilitate deployment.
Unresolved Matters and Future Directions
The experience in using the transcatheter approach to treat NAVR is limited but is expanding. Patients with mixed aortic valve disease with severe stenosis and at least moderate regurgitation have been successfully treated with both of the commercially available TAVR devices, but NAVR without stenosis is still considered a relative contraindication in published guidelines. Significant native aortic regurgitation is uncommon, and high-risk or inoperable patients with such pathology are few. Hence, the potential for this technology to expand exists, but is not of the same magnitude as with AS.
Newer-generation valve designs that use leaflet pinning or stabilization mechanisms are showing promise but experience with these devices is still limited. Larger registries, longer follow-up periods, and randomized clinical trials are necessary to gather sufficient evidence and experience to consider the widespread use of TAVR for NAVR.