Accumulating evidence suggests that tricuspid regurgitation (TR) is independently associated with reduced long-term survival in the community and patients with ischaemic or dilated cardiomyopathy.1 Patients with moderate-to-severe TR suffer symptoms of progressive right heart failure, which are often difficult to treat with diuretic therapy. Prevalence estimates based on data from the Framingham Heart Study suggest that TR affects approximately 1.6 million patients in the US; however, only 0.5 % of patients are currently estimated to undergo tricuspid valve repair or replacement, suggesting a large unmet need in treatment options for TR.2 In developed countries, functional or secondary TR is the most frequent aetiology of tricuspid valve disease (accounting for approximately 80 % of all TR cases).3 Functional TR is caused by tricuspid annular dilatation and leaflet tethering in the setting of right ventricular remodelling due to pressure or volume overload. In clinical practice, alterations of right ventricular geometry and function are most commonly due to pulmonary hypertension and left-sided heart disease. Relevant mitral valve disease is observed in more than 30 % of cases of functional TR.4 Particularly in older patients, functional TR is often associated with AF.5 In addition, transvenous placement of pacemaker and implantable cardioverter defibrillator leads may have adverse effects on tricuspid valve function.6
Challenges in the Treatment of Patients with Functional Tricuspid Regurgitation
Due to the common association with left-sided heart valve disease or cardiomyopathy, functional TR was merely considered as a bystander for a long time and management focused primarily on correction of the left-sided pathology.1 However, more recent data indicate that TR does not resolve following correction of left-sided heart valve disease.7 Instead, moderate preoperative TR represents a significant risk factor for severe TR on follow up after mitral valve surgery, and the presence of tricuspid annular dilatation at the time of mitral valve surgery is associated with adverse right ventricular remodelling and increasing TR severity on echocardiographic follow up.8,9 Nevertheless, functional TR still remains undertreated and patients are referred for intervention late in the natural history of the disease when tricuspid annular or right ventricular dilatation is advanced and right ventricular function is impaired. This entails that many patients with severe TR are deemed at very high or prohibitive surgical risk. On the other hand, current guidelines emphasize the fact that data from clinical studies addressing the question as to when and how TR should be corrected are limited. Both the 2012 version of the European Society of Cardiology/European Association for Cardio-Thoracic Surgery guideline, and the 2014 version of the American Heart Association/American College of Cardiology guideline for the management of patients with valvular heart disease provide a class I (level of evidence C) recommendation for tricuspid valve surgery in patients with severe TR undergoing left-sided valve intervention.10,11 Moreover, the guidelines provide a class I (level of evidence C) recommendation for surgery in symptomatic patients with severe isolated TR. A class IIa (level of evidence C) recommendation is given to patients with moderate primary TR and patients with mild or moderate secondary TR with dilated annulus (≥40 mm or >21 mm/m²) undergoing left-sided valve surgery.10,11 The latter recommendation justifying tricuspid valve surgery in patients with moderate or even mild TR appreciates the increased risk of progressive TR and right heart failure once tricuspid annular dilatation has occurred. In such instances, concomitant tricuspid valve repair can be performed without increased perioperative complication rates.12 In contrast, isolated reoperative tricuspid valve repair/replacement carries an excessively high operative risk.3 Compatible with this recommendation, a recent meta-analysis of fifteen studies including 2,840 patients suggested a reduction in cardiac-related mortality and improved echocardiographic outcomes for TR after a mean weighted follow up of 6 years in patients with concomitant tricuspid valve repair during left-sided valve surgery as compared with patients without concomitant tricuspid valve repair.13
In recent years a growing number of elderly patients with left-sided heart valve disease are treated by transcatheter valve technologies, and accumulating evidence suggests residual TR remains a predictor of adverse outcome in these patients. In patients with mitral regurgitation undergoing MitraClip® (Abbott Vascular) implantation, increasing TR severity correlates with higher one-year mortality and bleeding rates.14,15 Severity of TR in association with aortic stenosis is frequently progressive despite transcatheter aortic valve implantation.16 In addition, persistence of moderate or greater TR 6 months after transcatheter aortic valve implantation, but not preoperative TR severity, is associated with lower survival after adjustment for clinical variables and echocardiographic parameters of right ventricular function.16,17 Given the incidence and prognostic relevance of TR on the one hand and the challenges in providing treatment options on the other, there is increasing interest in the development of transcatheter approaches for treating TR.
Changes of Tricuspid Annular Geometry in Patients with Functional Tricuspid Regurgitation
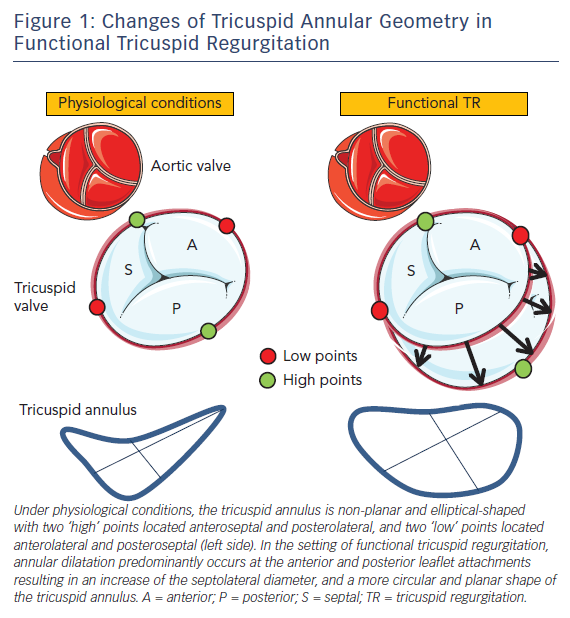
The tricuspid annulus is a complex and dynamic 3D structure that differs from the more symmetric saddle-shaped anatomy of the mitral annulus and is altered in the presence of different loading conditions of the right heart chambers.18 In addition, the tricuspid annular area changes significantly during the respiratory and cardiac cycle with an almost one-third increase during atrial systole and again in late systole/early diastole.19 Under physiological conditions the tricuspid annulus is elliptic or ‘egg’-shaped when imaged in a short-axis view (see Figure 1). Importantly, 3D-echocardiographic data suggest a normal tricuspid annulus is non-planar, exerting two high points (stretched towards the right atrium) anteroseptal adjacent to the aortic valve and posterolateral, as well as two low points (stretched towards the right ventricular apex) anterolateral and posteroseptal.20 The septal tricuspid leaflet arises directly from the tricuspid annulus above the interventricular septum and this medial portion of the tricuspid annulus is relatively fixed due to its position between the fibrous trigones, analogous to the intertrigonal portion of the mitral annulus.4 Therefore, the tricuspid annulus primarily dilates along the free wall, i.e. at the anterior and posterior leaflet attachments.7 In contrast to normal tricuspid annular geometry where the septolateral distance is larger than the anteroposterior distance, the tricuspid annulus becomes more circular and planar when functional dilatation occurs (see Figure 1). The displacement of the low and high points of the tricuspid annulus likely alters the papillary muscle-to-leaflet and annulus relationship, thereby contributing to leaflet tethering in functional TR.
Technical Difficulties in the Development of Transcatheter Therapies for Functional Tricuspid Regurgitation
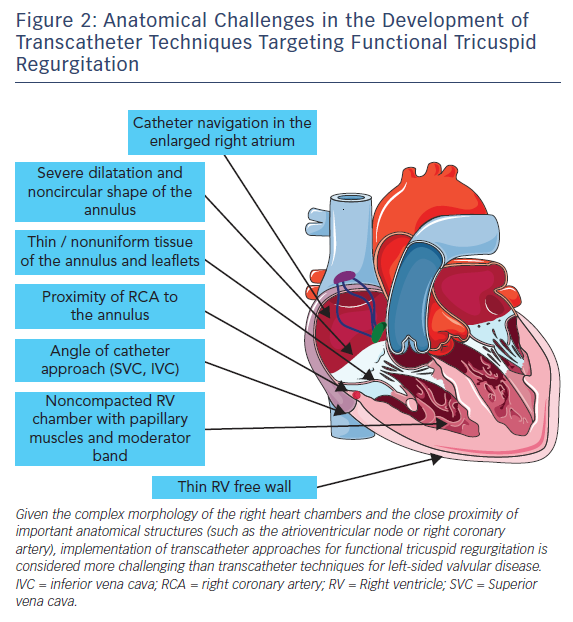
Several devices are currently in preclinical and early-clinical evaluation for transcatheter treatment of functional TR.21 However, the number of patients treated is still low and long-term haemodynamic and clinical benefits are unknown. As compared to interventional therapies targeted to the aortic or mitral valve, transcatheter treatment of TR has to face a number of additional anatomical challenges, as summarised in Figure 2. In particular, device steering via femoral access is hampered due to the sharp angle between the inferior vena cava and the tricuspid valve annular plane. Catheter navigation in the enlarged right heart chambers can be challenging. Application of annular-based devices has to account for the thin and non-uniform tissue of the tricuspid annulus and leaflets as well as the course of the right coronary artery.
Existing transcatheter therapies for functional TR can be divided into four groups according to their mode of action. The first group comprises percutaneous tricuspid valve annuloplasty devices. Among these are the TrialignTM system (Mitralign, Inc.), which will be described in detail below, as well as the Cardioband device (Edwards Lifesciences) and the TriCinchTM system (4Tech Inc), which reduces the septolateral tricuspid annular diameter by implanting a corkscrew anchor in the anteroposterior tricuspid annulus and applying tension on the annulus via a Dacron band fixed to a self-expanding nitinol stent in the inferior vena cava.22–24 The second transcatheter technique currently used to treat functional TR is tricuspid edge-to-edge repair using the MitraClip system with a modified steering technique.25 The third group comprises techniques for heterotopic caval valve implantation, where either two dedicated self-expandable bioprosthetic valves (TricValve [P&F Products & Features Vertriebs GmbH, in cooperation with Braile Biomedica]) or balloon-expandable valves used to treat aortic stenosis (29 mm Edwards SAPIEN XT or SAPIEN 3 [Edwards Lifesciences Corp]) are placed in the inferior and superior vena cava.26,27 The use of balloon-expandable valves requires prior implantation of a self-expandable stent in the inferior (and occasionally superior) vena cava to prepare a landing zone, given the large diameter of the vena cava and the hepatic vein confluence. Another device currently in clinical testing (FORMA Repair System, Edwards Lifesciences) aims to reduce malcoaptation of the tricuspid leaflets by placing a spacer through the central coaptation line, which reduces the regurgitant orifice area.28
Percutaneous Valve Annuloplasty: From Mitralign to Trialign
The Trialign device is a transjugular suture-based tricuspid valve annuloplasty system that reduces tricuspid annular diameter through tissue plication.29 The underlying transcatheter annuloplasty technique, which is known as the Mitralign device, was originally designed for the treatment of functional mitral regurgitation and first successfully applied in 2013.29 For mitral regurgitation, two pairs of sutured pledget implants are placed in the mitral annulus in the medial (P1) and lateral (P3) scallop regions via transfemoral access through the aorta across the aortic valve. Plication is achieved by advancing a dedicated plication lock device over the two sutures attached to each pair of pledgets and, finally, a stainless steel lock is placed to maintain plication of the mitral annulus.29 In 2016, Nickenig et al. reported the results of a safety, feasibility and efficacy study testing the Mitralign system in 71 patients with functional mitral regurgitation and high surgical risk.30 The device was successfully implanted in 50 of 71 patients (70.4 %) with no intraprocedural deaths or necessity for acute conversion to open heart surgery. Cardiac tamponade requiring pericardiocentesis occurred in 4 patients, likely related to catheter manipulation within the ventricle. During 6 months of follow up, non-urgent mitral valve surgery was performed in one patient (2.4 %) and non-urgent percutaneous mitral valve repair in seven patients (17.1 %). Although beneficial effects on left ventricular volumes, mitral valve annular geometry and leaflet coaptation were observed after 6 months of follow up, mitral regurgitation itself improved only in 50 % of treated patients. The percentage of patients presenting with New York Heart Association (NYHA) functional class ≥III was reduced by 56 % and the 6-min walking distance significantly increased during follow up by 56 metres.30 Further data on the performance of the Mitralign device in patients with functional mitral regurgitation are not yet available.
In 2015, Schofer et al. reported the first successful case of a direct percutaneous tricuspid valve annuloplasty in a patient with functional TR and prohibitive surgical risk using the Mitralign device with a modified delivery approach, a technique that is nowadays better known as the Trialign system.31 From a mechanistic point of view, this approach resembles a suture bicuspidization technique originally described by Kay et al.32 The Kay procedure aims to reduce TR by obliterating the annular segment corresponding to the posterior leaflet through placement of pledget-supported mattress sutures in the annulus. As a result, the tricuspid annular circumference is reduced and the tricuspid valve is converted into a smaller but competent mitral-like valve.32
Retrospective data suggest TR in patients treated by bicuspidization annuloplasty was zero to mild in 75 % at 3 years postoperatively, compared to 69 % in those undergoing ring annuloplasty.33 In a large retrospective series of 2,277 patients treated by various surgical techniques to eliminate TR, 81 % of patients treated by suture bicuspidization had zero or mild TR after 5 years of follow up, which compared well with other surgical techniques.34
Pre-procedural Assessment
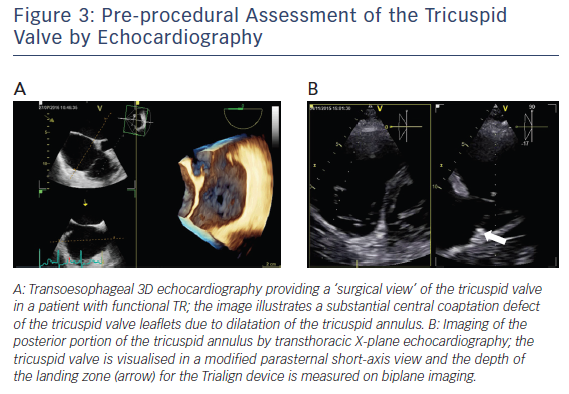
A comprehensive clinical, laboratory, echocardiographic and invasive evaluation is needed to assess whether a patient is suited for percutaneous tricuspid valve annuloplasty with the Trialign system. All patients treated so far were either enrolled in safety and performance studies or compassionate use cases. According to current study protocols, patients aged ≥18 and ≤80 years with moderate-to-severe chronic functional TR are considered symptomatic despite guideline-directed medical therapy (i.e. NYHA class II, III or ambulatory IV) are considered. A heart team decision is needed to rule out the need for left-sided valve surgery and to decide whether tricuspid annuloplasty would be suited for the patient. Transthoracic and transoesophageal 2D/3D echocardiography is central to the evaluation of percutaneous tricuspid valve annuloplasty with the Trialign system, including deep oesophageal and transgastric imaging of the tricuspid valve (see Figure 3A). Current eligibility criteria for the Trialign procedure include a left ventricular ejection fraction ≥30 %, tricuspid annular plane systolic excursion (TAPSE) ≥13 mm and systolic pulmonary artery pressure (sPAP) ≤60 mmHg. Furthermore, emphasis needs to be paid on the tricuspid valve annular diameter (≥40 mm [or 21 mm/m2] and ≤55 mm [or 29 mm/m2]), tricuspid effective regurgitant orifice area (EROA; ≤1.2 cm2) and sufficient posterior annular dimension for device implantation. The latter is best visualised on transthoracic X-plane imaging of the tricuspid valve in a parasternal short-axis view (see Figure 3B).
Procedural Details on the Trialign System
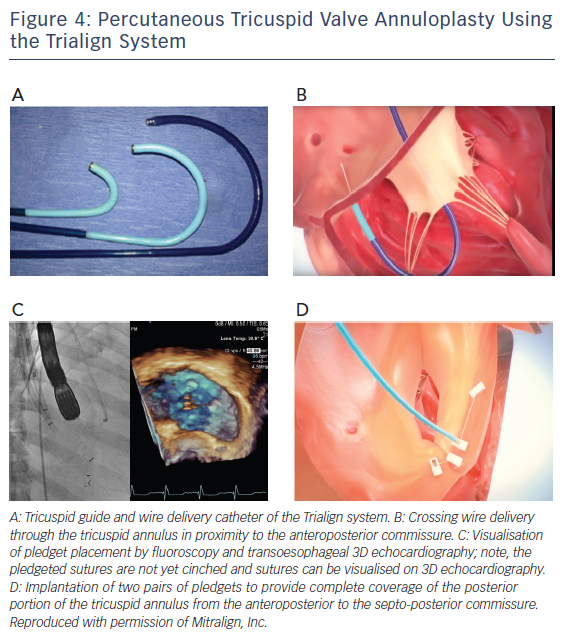
During the Trialign procedure a pair of polyester pledgets are delivered across the tricuspid annulus in proximity to the anteroposterior and septo-posterior commissure, cinched by a polyester suture to obliterate the posterior tricuspid leaflet, and locked on the atrial side (see Figure 4A).31,35
Access
Percutaneous tricuspid valve annuloplasty with the Trialign system requires two 14 F sheaths placed in the ventral and lateral portion of the right internal jugular vein with a distance of approximately 2 cm to avoid bleeding complications. In addition, a guide catheter needs to be placed in the right coronary artery via femoral access because of the close proximity of the right coronary artery to the posterior portion of the tricuspid annulus.
Crossing Wire Delivery
As a first step, a dedicated deflectable tricuspid guide catheter (see Figure 4A) is advanced into the right ventricle via jugular access.31,35 The tricuspid guide catheter is designed to direct all subsequent procedural devices across the tricuspid valve orifice and towards the tricuspid annulus by advancement/retraction, deflection and rotation. Next, a tricuspid wire delivery catheter is advanced to access the ventricular side of the tricuspid annulus at the target location for pledget delivery. With the help of transoesophageal 2D/3D echocardiography and fluoroscopy, the tricuspid wire delivery catheter is directed to the septo-posterior location. Then, a crossing wire is advanced through the lumen of the tricuspid wire delivery catheter. The crossing wire is used to cross the annulus from the ventricular to atrial side, thereby providing a path for pledget delivery through the tricuspid annulus. It is connected to a radiofrequency energy source and radiofrequency energy is employed at the distal tip while moving the crossing wire through the tricuspid annular tissue (see Figure 4B). Afterwards, the position of the crossing wire along the tricuspid annulus and the annular depth is confirmed by echocardiography.
Snaring
Once the crossing wire is properly placed at the septo-posterior portion of the tricuspid annulus the distal tip of the crossing wire is trapped in the right atrium by an endovascular snare system and retracted through the jugular access site.
Pledget Delivery
Following snaring of the crossing wire, a tricuspid pledget deliver catheter is positioned in the right atrium and the deflected tricuspid guide catheter is positioned under the tricuspid annulus in the right ventricle. Next, the pledget delivery catheter is advanced through the tricuspid annulus into the tricuspid wire delivery catheter. The pledget delivery catheter seats the distal portion of the pledget on the ventricle side of the tricuspid annulus. Afterwards, the pledget delivery catheter is retracted back through the annulus to the atrial side and deploys the proximal portion of the pledget.31,35
Second Pledget Delivery
The second pledget is positioned next to the anteroposterior commissure of the tricuspid annulus following the same technique as described for the septo-posterior location. A distance of 25–28 mm between the pledget in the anteroposterior and septo-posterior location is recommended (see Figure 4C). Of note, to provide complete coverage of the posterior portion of the tricuspid annulus from the anteroposterior to the septo-posterior commissure, investigators have tried to implant two pairs of pledgets (see Figure 4D). Whether the two-pair strategy is superior to implantation of a single pair of pledgets has not yet been tested.
Plication, Lock and Cut
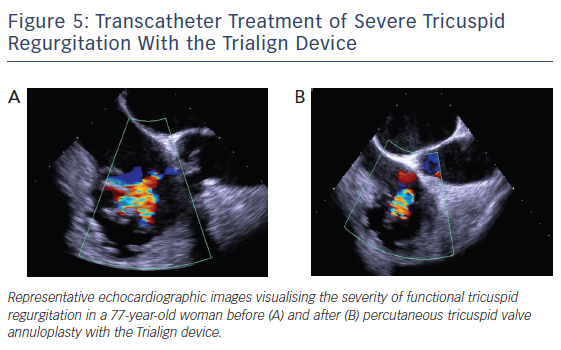
A dedicated plication lock delivery catheter is advanced over both pledget sutures to the atrial side of the tricuspid annulus. By advancing the plication lock delivery catheter towards the annulus, the portion of the tricuspid annulus and posterior tricuspid leaflet in-between the two pledgets is plicated. To secure plication the pledget delivery catheter deploys a lock onto the sutures. Finally, a suture cutter catheter is tracked over the locked sutures to the lock implant. The catheter tip encloses a blade that cuts both sutures right above the lock and allows removal of the proximal portion of the sutures. Representative echocardiographic images of a patient treated with a single pair of pledgets are shown in Figure 5.
Early Clinical Data on Safety and Performance
Percutaneous tricuspid valve annuloplasty with the Trialign system is in early clinical evaluation, and data on the safety, feasibility and clinical benefit are still limited. The first-in-human report of a direct transcatheter tricuspid valve annuloplasty using the Trialign system was published in 2015 by Schofer et al.31 An 89-year-old woman with recurrent right heart decompensation and massive TR (effective EROA 1.35 cm², tricuspid annular area of 14.1 cm2) underwent implantation of a single pair of pledgets, resulting in a 57 % reduction in tricuspid annular area and a 53 % reduction in effective EROA. Of note, right atrial pressure was reduced from 22 to 9 mmHg, and echocardiography revealed a profound increase in left ventricular stroke volume from 42 to 72 ml following the procedure.31
After publication of the first successful case of transcatheter treatment of functional TR with the Trialign device, another report emphasized the possibility of early failure due to pledget dehiscence.36 Recently, Hahn et al. reported the results of the Early Feasibility of the Mitralign Percutaneous Tricuspid Valve Annuloplasty System Also Known as Trialign (SCOUT) trial (NCT02574650), constituting the first report of an early feasibility trial for a transcatheter tricuspid valve device.35 The SCOUT trial is prospective, single-arm, multicentre, early feasibility study of the Trialign device and recruited 15 symptomatic patients (i.e. NYHA functional class ≥II) with moderate or greater functional TR. Detailed echocardiographic and quality-of-life measurements (i.e. NYHA functional class, Minnesota Living with Heart Failure Questionnaire and 6-min walk test) were performed at baseline and 30 days. Of note, all patients underwent successful device implantation with no serious complications. In one patient, coronary angiography after pledget delivery and lock showed tenting of the distal right coronary artery in the region of the plication with significant narrowing, ST-segment elevations on ECG and a fractional flow reserve of 0.57, requiring stent implantation. On follow up after 30 days, three patients had echocardiographic evidence of a single pledget detachment from the annulus.35 Significant reductions in tricuspid annular diameter and EROA were observed in the remaining patients. Left ventricular ejection fraction and right ventricular function (as measured by TAPSE) remained unchanged on echocardiography 30 days after the procedure. Despite a reduction of TR in the as-treated population (i.e. patients without pledget detachment) no significant difference in estimated sPAP was observed on echocardiography.35 Interestingly, left ventricular outflow tract stroke volume as measured by Doppler echocardiography increased following TR treatment with the Trialign device. Finally, reduction of TR in the intention-to-treat cohort translated into improvements in NYHA functional class, Minnesota Living with Heart Failure Questionnaire score and 6-min walking distance.35 The ongoing Safety and Performance of the Trialign Percutaneous Tricuspid Valve Annuloplasty System (SCOUT-II) study is a prospective, single‐arm, multicentre study in Europe and the US recruiting patients with moderate-to-severe TR in whom left‐sided valve surgery is not planned. SCOUT-II further investigates safety and performance of the Trialign system with follow up planned up to 2 years and is a European CE-Mark study.
Conclusion
Moderate-to-severe TR is a common problem in clinical cardiology and is associated with significant morbidity and reduced long-term survival. Several transcatheter techniques are currently in preclinical and early clinical evaluation as a novel treatment option for patients with symptomatic functional TR and high surgical risk. The Trialign device is a novel percutaneous tricuspid valve annuloplasty technique targeting the pathophysiologic hallmark of functional TR, i.e. dilatation of the tricuspid annulus along its posterior portion. By delivering and cinching two pledgeted sutures the posterior portion of the tricuspid annulus and the posterior tricuspid leaflet is plicated. The SCOUT trial has confirmed the safety and feasibility of the device. However, clinical data are still limited, and further studies are needed to confirm these data in larger cohorts of patients and to investigate whether applying the Trialign device leads to clinical benefit in patients with functional TR.