Percutaneous revascularisation has become the cornerstone of ischaemic heart disease management.1,2 Historically, coronary angiography and intervention was predominantly performed via the common femoral artery.3 However, this procedure has an associated 1.5–9.0 % risk of complications, most of which are related to bleeding at the femoral access site.4 Despite a significant reduction in the incidence of major femoral bleeding complications during 1994 to 2005 from 8.4 % to 3.5 %, respectively,5 related to technological advancement (including a reduction in size of interventional devices and, controversially, the introduction of vascular closure devices), these major complications remain important.6–9 Multiple large studies have demonstrated a two- to eightfold increase in mortality rate in patients with acute coronary syndrome (ACS) who experienced major bleeding following percutaneous coronary intervention (PCI).5,10–13
Campeau reported the first contemporary use of transradial access for diagnostic procedures in 1989;14 this was shortly followed by reports of the first transradial angioplasty.15,16 Several early studies reported a significant reduction in vascular complications for transradial procedures compared with the transfemoral approach.17–19 These early studies raised interest in the transradial access site as a viable and attractive alternative to femoral access.20–22 There followed larger, multicentre, prospective, randomised trials designed to overcome any potential bias of earlier single-centre trials and retrospective meta-analyses.
The Radial Versus Femoral Access for Coronary Angiography and Intervention in Patients with Acute Coronary Syndromes (RIVAL) study set out to determine whether radial access was superior to femoral access in patients with ACS undergoing coronary angiography and angioplasty.23 This large randomised, worldwide multicentre trial of 7,021 patients demonstrated that transradial procedures were associated with a 60 % reduction in vascular complications when compared with the femoral approach, but no significance difference in rates of death, MI, stroke or major bleed. However, the RIVAL primary PCI (PPCI) in ST elevation MI (STEMI) sub-analysis found that the radial artery approach was associated with a significant reduction in the rate of 30-day all-cause mortality.24
These findings led to the Minimizing Adverse Haemorrhagic Events by Transradial Access Site and Systemic Implementation of AngioX (MATRIX) trial, a large randomised, multicentre, superiority trial comparing transradial versus transfemoral approach in 8404 patients with ACS.27 The study found no reduction in the rate of MI, stroke, or non-coronary artery bypass graft-related major bleeding at 30 days, but a 63 % reduction in the risk of vascular-access complications was seen in the transradial group. The transradial approach was also found to reduce net adverse clinical events, and all-cause mortality and major bleeding rates. This reduction in the rates of allcause mortality and bleeding occurred in all patients with ACS and was not limited to patients with STEMI, as in the aforementioned RIVAL study.
Several early studies reported a reduction in mortality rates in patients undergoing transradial access for STEMI.28–31 These studies paved the way for the Radial Verses Femoral Randomized Investigation in ST elevation Acute Coronary Syndrome (RIFLE-STEACS) trial.
This prospective, randomised study evaluated transradial versus transfemoral arterial access in patients with STEMI. The study enrolled 1,001 patients across four Italian centres and found not only a 47 % reduction in the rate of access-site-related bleeding complications, but also a reduction in the rate of cardiac death and hospital stay with transradial procedure.25 These findings were verified by a meta-analysis of 12 studies including 5,055 patients recommended radial approach for patients with STEMI undergoing PPCI.26
The reduction in vascular complications has also been highlighted in other potential high-risk groups including obese patients,32 octogenarians33,34 and female patients.35
Further advantages of a transradial approach include immediate ambulation, as opposed to bed rest after femoral procedures. Reduced post-procedure nursing care, reduced hospital stay and, therefore, cost, with an overwhelming patient preference for transradial angiography are all well-described additional advantages.36–42
Findings from these studies have led to the recommendation for use of the radial artery approach in both patients with STEMI43 and non-STEMI.44
Opponents of radial access have cited an associated learning curve45 with adopting the transradial approach resulting in longer procedural time and increased radiation exposure.46 However, a meta-analysis found the difference in radiation exposure between transradial and transfemoral approaches to decrease by >75 % over a 20-year period, and that the clinical benefits of transradial access outweighed any small observed difference in radiation exposure.47 In addition, higher-volume radial operators were shown to have shorter procedural and fluoroscopy times. Lower-volume operators achieve a reduction in procedural and fluoroscopy times as their procedural experience increases.48 Similarly, a sub-analysis of the multicenter RIVAL study found no significant differences in radiation exposure between either femoral or radial access for the entire cohort. However, a modest, but significant, increase in fluoroscopy time in radial cases performed in a low- to intermediate-volume center, but not in high-volume centers. Furthermore, a sub-analysis and multivariate analysis found the highest radial volume centres and operators had the lowest radiation exposure.49
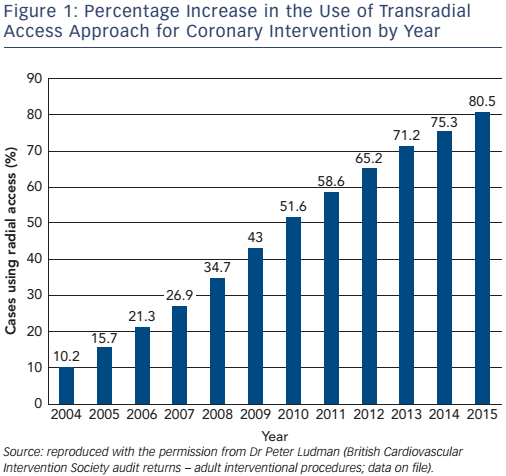
Finally, Burzotta et al. found that the case volume required to overcome the learning curve was relatively short – 50–80 transradial procedures.50 As a result of these studies, operators in the UK are increasingly adopting radial access (see Figure 1).22
Tips for Successful Transradial Coronary Artery Procedures
Know Your Patient
The first tip begins outside the catheter laboratory. A full and detailed explanation of the procedure should be provided not only as a consenting exercise, but also to decrease patient anxiety. It is important to obtain details of any significant patient comorbidity, allergies, previous coronary artery bypass grafts and or PCI. In patients with previous PCI, it is important to determine which arterial access approach was used, and if any difficulties were encountered including switching access site or post-procedural bleeding. Previous angiographic images should be reviewed, if possible, to identify any pre-existing coronary artery disease or procedural difficulties encountered, and to ascertain the presence of patent grafts and any vascular abnormalities or tortuous vasculature. Relevant blood tests including haemoglobin, renal function and troponin levels should also be reviewed. The Allen’s test, once thought to be pivotal in the assessment of patiens suitability for radial angiography, is now recognised to be of little value due to ulnar–palmer collateralisation.51 Therefore, the routine use of Allen’s test is no longer recommended within the author’s institute.
Venous Access
All patients should have intravenous access, preferably in the contralateral arm to the side of transradial approach, allowing administration of medication including intravenous saline or sedatives. Radial artery spasm has become an increasingly recognisable phenomena52 seen in 10–15 % of reported cases of transradial procedures.53,54 Catecholamine release due to anxiety increases risk of radial artery spasm; therefore, the patient should be as relaxed as possible. Sedation has often been used in attempt to prevent radial artery spasm. A study of 2,013 patients who were randomised to receive fentanyl plus midazolam or no sedation found a significant reduction in spasm, and the number needed to treat to avoid one case of radial artery spasm was 18.55 The crossover rate to femoral artery was 34 % lower in the group given fentanyl and midazolam. The results of this study supported the use of pre-procedural sedation. However, the study was criticised for incomplete reporting.56 A large multicentre and worldwide study found not only a wide geographic variation in the use of sedation, but also that 58.3 % of operators did not routinely use sedation.21 At present, there are insufficient data to recommend routine use of sedation.
Left or Right Radial Artery Access
Transradial angiography and PCI are predominantly performed from the right radial artery due to cardiac catheter laboratory set, operator comfort and preferences.
Traditionally, there had been concerns about radiation dose and success rates of the left radial approach. However, the (Randomized Evaluation of Vascular Entry Site and Radiation Exposure) REVERE trial found no significant difference in radiation dose in 1,500 patients undergoing either femoral, right or left radial artery approaches.57 The study also found a reduction in radiation dose in experienced radial and femoral operators.58,59 These studies also found no difference in contrast load, number of catheters used or success rates.58,59 Major adverse cardiac and cerebrovascular event rates have also been found to be similar.58,59 Norgaz et al. attributed shorter fluoroscopy times from left radial artery approach to a threefold higher incidence of subclavian tortuosity, as well as a higher incidence of radial loops with right radial access.59
Further advantages of using the left radial artery approach include significantly shorter learning curve and progressive reduction in fluoroscopic and arterial cannulation times when compared with right radial artery approach.60 Therefore, the left radial approach is both feasible and safe in clinical practice.
The left radial artery approach may also be used post coronary artery bypass graft if patients have had a left internal mammary artery (LIMA) graft to the left anterior descending (LAD) artery. This is because cannulation of the LIMA to LAD graft has been associated with a failure in 27 % of cases if performed from the right radial artery.61 The presence of a retro-oesophageal origin may either preclude or render more complex cannulation of the coronary arteries from the right radial artery, whereas a left radial approach may prove to be easier and more successful. The patients arm may be placed on an arm board anchored under the patient either at 80° or alongside the patient depending on operator preference. A folded bed sheet or specialised arm board devices placed under the wrist allows hyperextension of the wrist. This allows not only increased support, but also exposure of the radial artery. The wrist is then cleaned, draped and infiltrated with local anaesthesia.
Radial Artery Puncture Kits and Spasmolytics
A number of radial access kits are currently commercially available, including the bare-metal Micropuncture® system (Cook Medical) and a Glidesheath Slender hydrophilic-coated introducer sheath (Terumo). The choice of puncture kit is at the discretion of the operator; however, familiarity of both kits is advisable. Irrespective of the puncture system used, the radial artery should be punctured at 30–45° to the horizontal and 2 cm proximal to the radial styloid process, to minimise the risk of introducing the sheath into a smaller diameter distal radial artery. A small skin incision may be performed while the guide wire is in situ, allowing easier introduction of the sheath and further reducing any distress or pain experienced by the patient. Once the radial sheath has been introduced spasmolytics are often administered to try to prevent radial artery spasm.62 A meta-analysis found that 5 mg of verapamil or verapamil in combination with nitroglycine had the lowest rates of radial artery spasm.54 In a survey across 75 countries, the majority (85.9 %) of operators used vasodilators prophylactically with verapamil being the most commonly used agent (75.3 %), either alone or in combination with a number of other agents.21
The use of prophylactic vasodilator anti-spasmolytic cocktails is largely operator preference based on the operator’s own common practice rather than based on rigorous placebo-based clinical trials. The rate of radial artery spasm varies widely in different trial and this may be attributed due to a lack of consensus of the definition of radial artery spasm. This, in turn, limits inter-trial comparisons and, therefore, any meta-analysis. However the use of anti-spasmolytic agents have been questioned. Technical advancements such as a reduction in the diameter and the addition of hydrophilic coating of radial introducer sheaths both reducing risk of radial artery spasm. Geographic variation of the use of anti-spasmolytics has been observed, and up to 72.2 % of Japanese operators do not use anti-spasmolytics.21 The study results indicate that the preventative use of anti-spasmolytics may not be as important as once thought.
Radial artery spasm has been found to be a rare event after a learning curve (1.7 %) and use of verapamil 5 mg showed no significant difference in investigated endpoints, including access site conversion, radial artery spasm or subjective pain, compared with placebo.63 These findings led to further questioning of the use of verapamil in high-volume operators. The authors concluded that prophylactic vasodilators showed no advantage.63 The omission of vasodilators may be clinically relevant by avoiding adverse effects and not precluding radial angiography in patients with known contraindications to vasodilators.64
Radial Artery Anomalies and Tortuosity
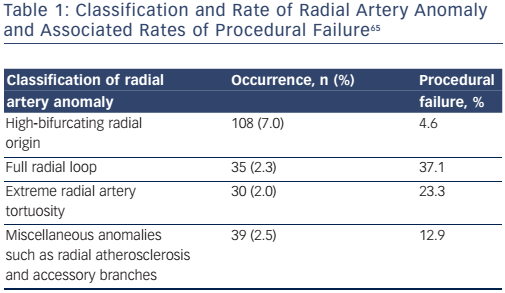
A landmark study of 1,540 consecutive patients found that radial artery anatomy anomalies are a relatively common finding occurring in 13.8 % (n=212) of patients undergoing transradial coronary procedures.65 Radial artery anomalies were associated with a significant incidence of procedural failure (14.2 versus 0.9 % in non-anomalous radial arteries; see Table 1). Despite this, the overall procedural success was found to be 96.8 %, with only 1 % (n=5) having vascular complications, all managed conservatively without any ischaemic sequelae. The authors, therefore, recommended imaging of the radial artery after introducing sheath insertion.
Certain radial artery anomalies (such as large-diameter radial loops) may be difficult to overcome and an alternative vascular access may be required. However, there are several available techniques for overcoming simpler anomalies such as radial tortuosity. Balloonassisted tracking is a technique that may be used to overcome radial tortuosity, spasm or loops.66,67 A regular 0.014” hydrophilic coronary angioplasty wire is passed through the difficult area under fluoroscopy. Then a diagnostic or guide catheter is loaded with a standard non-compliant balloon positioned half protruding beyond the tip of the catheter. A 5 Fr catheter will accommodate a 2.0 mm balloon, whereas a 6 Fr guide may require a 2.5 mm diameter balloon. Once correctly positioned, the balloon is inflated to 8–10 atmospheres. The catheter–balloon delivery system is then loaded onto and passed along the 0.014” hydrophilic coronary angioplasty wire. The balloon– catheter delivery system creates a soft tapered edge straightening the radial tortuosity and facilitating catheter passage through the loops or areas of spasm, limiting further radial trauma and pain. This manoeuvre is performed under fluoroscopic guidance (see Figure 2). Once the catheter has reached the ascending aorta the 0.014” hydrophilic coronary angioplasty wire and balloon catheter may be changed to a standard 0.035” guide wire, providing greater support to position the catheter into the aorta root. An exchange length J-tip (260 cm) guidewire is then used to exchange catheters to avoid loss of radial access.
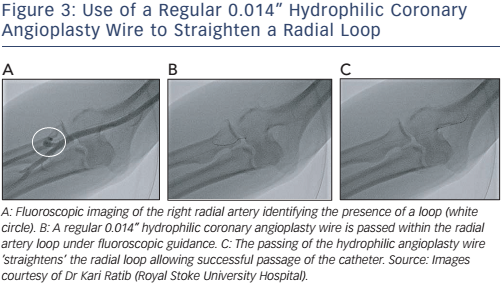
A simpler method is passing a regular 0.014” hydrophilic coronary angioplasty wire cautiously along the tortuosity and secured within the subclavian artery. This often straightens out the radial artery. A 5 Fr diagnostic multipurpose catheter is then loaded onto the proximately positioned wire. The multipurpose catheter decreases the risk of the radial artery wall shearing and the damage that occurs with more angulated catheters such as the Judkins or Amplatzer® catheters. Cautious advancement under fluoroscopic guidance is mandatory. First, to avoid engagement into small branches and to ensure the catheter is smoothly traversing to the head and neck vessels and, second, to prevent catheter kinking within the radial artery causing intense spasm and pain to the patient (see Figure 3A–D). Once the catheter has reached the subclavian artery just proximal to the end of the regular 0.014” hydrophilic coronary angioplasty wire, the coronary angioplasty wire is then exchanged with a standard J-tip (260 cm) exchange wire. The exchanged wire provides greater support for the catheter. The multipurpose catheter is then withdrawn ensuring the exchange length (260 cm) guidewire remains positioned within the subclavian artery. A standard diagnostic or guide catheter may then be loaded onto the J-tip exchange length (260 cm) guidewire, and both passed under fluoroscopic guidance to the aortic root in standard manner. The coronary arteries are then cannulated. All subsequent catheter changes are performed via the J-tip (260 cm) guidewire avoiding the need to re-cross areas of difficult anatomy.
Similarly, the above techniques may also be applied in the presence of tortuous brachial or subclavian arteries. Deep inspiration with breath holding may allow further negotiation of subclavian artery tortuosity by modifying the angulation of the brachiocephalic trunk. The optimal view for assessing the ascending aorta is in the left anterior oblique projection at 30°. This projection limits superposition of different segments of the aorta and opens out the aortic arch. Recognition of the position of the guidewire in either ascending or descending aorta is then made possible. If the guidewire repeatedly enters the descending rather than ascending aorta a diagnostic 5 Fr or 6 Fr JR4 catheter may be advanced with great care not to extend beyond the guidewire. On reaching the aorto– brachiocephalic or aorto–subclavian junction for right and left radial artery access, respectively, the catheter can be angulated towards the ascending aorta facilitating wire access. An exchange length (260 cm) guidewire should be used if further catheter exchanges are required.
Finally, all catheters should always be withdrawn over a 0.035” guidewire even in non-tortuous radial arteries. This manouvre avoids any forceful manipulation or catheter tip induced trauma that may cause catheter kinking and radial artery spasm or avulsion.68,69
Radial Artery Diameter
Radial artery diameter may potentially limit the maximum size of radial artery introducer sheath, especially as the external diameter of the introducing sheath is 2 Fr larger than its internal diameter.70 The ideal ratio of inner diameter of radial artery to sheath outer diameter has been found to be 0.9.71 Operators should avoid using sheath diameters greater than the radial artery diameter.72 The smaller-diameter hydrophilic introducer sheaths are associated with a reduction in both incidence of radial artery spasm73,74 and pain experienced by patients.71 Therefore, radial artery access had been thought to preclude larger-bore guide catheters required for more complex lesions.
There are several approaches that may overcome this potential technical challenge. The first is the use of the Glidesheath Slender® (Terumo) introducer sheath, which has a thin wall providing an inner diameter compatible with 6 Fr catheters and an outer diameter corresponding to 5 Fr sheath, allowing passage of large-bore guide catheters. These introducer sheaths have been reported to have high success rates with a significant reduction in radial artery occlusion and radial artery spasm.75,76
Sheathless guide catheters negate the use of radial introducer sheaths. This technique has been shown to be both safe and effective in both elective and primary PCI for patients with STEMI.77 However, the main advantage is the ability to allow transradial passage of the large-bore 7 or 8 Fr guide catheters that may be required for complex coronary interventions.70 The radial artery is cannulated with a standard 5 or 6 Fr radial artery sheath. Diagnostic coronary angiography may be performed in the usual maner with either 5 or 6 Fr diagnostic catheters. If diagnostic images indicate that coronary intervention is required with a large-bore guide catheter then the 5 or 6 Fr diagnostic catheter is removed over a J-tip (260 cm) guidewire positioned and secured in the ascending aorta. The introducer sheath is then also removed cautiously over the J-tip (260 cm) guidewire. Pressure is then applied onto the radial artery access site once the introducer sheath is removed. A 7 or 8 Fr standard guide catheter with a 5 Fr multipurpose catheter extending beyond the tip provides a smooth transition from wire to catheter, and is loaded on to the J-tip (260 cm) guidewire. This delivery system is then passed into the ascending aorta under fluoroscopic guidance. The inner 5 Fr multipurpose catheter is then removed leaving the 7 or 8 Fr guide within the ascending aorta ready to be manoeuvred in the standard way to cannulate the coronary artery. On completion of the intervention, the guide catheter is taken out using the standard over-the-wire technique and a haemostatic compression device is placed.78
A modified version adopts the balloon-assisted technique described above for tortuous radial artery. A regular 0.014” hydrophilic coronary angioplasty wire is passed under fluoroscopic guidance through a standard 5 or 6 Fr radial introducer sheath to the ascending aorta. The introducer sheath is removed over the hydrophilic coronary angioplasty wire. A 7 Fr introducer without the sheath is loaded and passed along the coronary angioplasty wire to ensure a passage has been made into the radial artery. The delivery system is then loaded onto and passed along the hydrophilic coronary angioplasty wire to the ascending aorta. The delivery system consists of a largebore guide catheter with a balloon catheter positioned so that it protrudes partially outside the guide catheter. The balloon is then inflated to low pressure, 6–8 atmospheres. The delivery system creates a soft tapered edge that passes within the radial artery. Once the delivery system has reached the ascending aorta the balloon is deflated and, along with the 0.014” hydrophilic coronary angioplasty wire, may be changed to a standard 0.035” guidewire providing greater support. The coronary artery is then cannulated the standard way.
Radial artery occlusion
One of the advantages of the radial artery is its superficial location, which allows safe and effective haemostasis by compression. This has led to many haemostatic compression devices becoming available, including TR Band ® (Terumo), RadiStop™ (St. Jude Medical), RADstat® (Merit Medical Systems) and Helix® band (Vascular Perspectives). The most frequent complication of radial procedures is radial artery occlusion (RAO). The technique of patent haemostasis has been shown to significantly reduce radial artery occlusion at 30 days and, in the opinion of the authors, should be standard practice.79,80 RAO is usually asymptomatic due to ulnar–palmar collateralisation vascular blood supply of the hand. However, RAO precludes the use of radial artery access in any future coronary interventions. Procedural duration, arterial diameter-to-sheath ratio, compression time and pressure all have been shown to be risk factors for RAO.80 Heparin has been shown to significantly reduce rates of RAO, with a linear relationship observed between heparin dose and rate of RAO.81 This has led to most operators administering 5,000 IU of heparin or 70 IU/kg intra-arterial via the radial sheath. Heparin may also be given intravenously, with no difference in RAO whether given intra-arterially or intravenously.82 However, there are no current recommendations on heparin dose in patients taking oral anticoagulation or receiving platelet glycoprotein IIb/IIIa inhibitors (abciximab, eptifibatide, tirofiban) or direct thrombin inhibitor use (bivalirubin).
Finally, radial artery spasm has also been identified as a potential risk factor for RAO.73 However, a meta-analysis found no data to assess any link between pharmacological prevention of RAS and prevention of RAO, further highlighting the importance of preventing RAS.54
Conclusion
There has been an exponential growth in the use of transradial coronary artery procedures over the last two decades. This increased use of transradial procedures has led to a number of potential technical challenges becoming recognised. However, with increasing experience many new approaches are now becoming available to overcome these potential challenges to transradial coronary procedures.







