Obstructive unprotected left main coronary artery (ULMCA) disease has been recognised as a high-risk condition since the 1960s owing to the large quantum of myocardium supplied by this system (see Figure 1). In that era, medical therapy was the only available management and 5-year mortality rates exceeded 60 %.1 With the introduction of coronary artery bypass grafting (CABG) it became clear that surgical management of these patients conferred a significant mortality benefit over medical therapy alone.2,3 For several decades CABG was accepted as the first line management option for ULMCA disease while techniques for the percutaneous management of coronary atherosclerosis were in the early stages of development. While percutaneous transluminal coronary angioplasty was deemed too high risk in this setting, the introduction of bare-metal stents (BMS) in 1986 marked the beginning of the era of percutaneous management of ULMCA disease.4 Early results of BMS use were mixed with intraprocedural mortality rates as high as 10 % for ULMCA interventions and 3-year survival rates of 30 %.4 In-stent restenosis was also identified as a significant barrier to the adoption of BMS in this patient population, with mortality rates as high as 40 % reported. Drugeluting stents (DES) were developed primarily to tackle the high rates of in-stent restenosis being observed, and numerous studies confirmed the overall safety and long-term patency of DES. Advances in technique and stent design combined with newer antiproliferative drugs led to the increasing adoption of percutaneous coronary intervention (PCI) in the management of ULMCA disease in the mid-2000s.
In the context of increasing adoption of PCI to treat ULMCA disease, the need for robust clinical trials comparing PCI to CABG arose. The Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (SYNTAX) trial sought to determine the optimal revascularisation strategy for patients with multivessel coronary artery disease or ULMCA disease with randomisation to either CABG or PCI with paclitaxel-eluting (TAXUS, Boston Scientific) stents.5 The SYNTAX trial was a prospective, multicentre, randomised clinical trial that successfully randomised 1800 patients to CABG or PCI groups and enrolled 1275 patients in nested registries for PCI or CABG, as they were not suitable candidates for both procedures. The trial was designed to test the non-inferiority of PCI compared to CABG. The primary endpoint was a composite of death (all cause), stroke, MI or repeat revascularisation. Over a period of 12 months the primary endpoint occurred in 17.8 % of patients in the PCI group versus 12.4 % in the CABG group, which failed the prespecified non-inferiority margin. The observed difference in primary endpoint was primarily driven by a higher repeat revascularisation rate in the PCI group (13.5 % versus 5.9 %) and no difference was seen in all-cause death rate or the combined endpoint of all-cause death, MI and stroke. Thus, PCI was deemed to be inferior to CABG in the SYNTAX trial for management of multivessel coronary artery disease.
Subgroup analysis of the ULMCA group (n=705), while considered strictly hypothesis generating given the failure of the primary outcome, painted a more nuanced picture.6 No difference was observed for the primary composite endpoint between the CABG and PCI groups (13.7 % and 15.8 %, p=0.44) in this population. PCI appeared numerically favorable in regard to major adverse cardiac and cerebrovascular events (MACCE) in isolated ULMCA disease (7.1 % versus 8.5 %), left main plus concomitant single vessel disease (7.5 % versus 13.2 %) and those with low or intermediate SYNTAX scores (≤32). CABG was favored in those with two or three vessel disease in regard to MACCE. Overall, PCI MACCE rates were driven largely by increased repeat revascularisation (6.5 % versus 11.8 %; 95 % CI [1.0–9.6], p=0.02), while the stroke rate in the CABG group was considerably higher at 1-year follow-up (2.7 % versus 0.3 %; Δ−2.4 %; 95 % CI [−4.2–−0.1], p=0.009). Thus, the SYNTAX trial suggested that in patients with ULMCA disease, especially those with low complexity lesions as identified by SYNTAX scores ≤32, PCI could be a reasonable alternative to CABG with the benefit of fewer strokes at 12 months being balanced by higher rates of repeat revascularisation.
The final results of the left main subgroup within the SYNTAX trial were reported at 5-year follow-up and confirmed the 1-year outcomes with no difference in MACCE between the PCI and CABG groups (36.9 % versus 31.0 %, p=0.12) in the ULMCA disease subgroup.7 When analysed by SYNTAX score, MACCE in the high-risk group (≥33) was significantly higher with PCI (46.5 % versus 29.7 %, p=0.003) and repeat revascularisation rates remained higher in the PCI group irrespective of SYNTAX score (26.7 % versus 15.5 %, p<0.01). While mortality was no different in the two groups at 5 years, the benefit of PCI in regard to lower stroke incidence persisted at 5 years (1.5 % versus 4.3 %, p=0.03). These findings were largely confirmed by smaller scale randomised Left Main Coronary Artery Stenting (LE MANS) and Premier of Randomized Comparison of Bypass Surgery versus Angioplasty Using Sirolimus-eluting Stent in Patients with Left Main Coronary Artery Disease (PRECOMBAT) trials as well as meta-analyses of high-quality studies completed at the time.8–11
In the background of the SYNTAX trial, non-randomised single-centre and registry-based studies were also underway, the largest of which was the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization (MAIN COMPARE).12 This cohort study enrolled 2240 patients from 12 centres in Korea with a minimum follow-up of 3 years with patients undergoing CABG or PCI with BMS early on and subsequently first- and second-generation (sirolimus) DES. Overall, no difference was noted between the two groups in regard to death or a composite endpoint of death, MI or stroke. However, target vessel revascularisations were significantly higher in the PCI group (HR 4.55; 95 % CI [2.88–7.20], p<0.001).
Target vessel revascularisation (TVR) emerged as a constant signal that was driving higher MACCE rates in ULMCA disease treated with PCI in both randomised trials and cohort studies. Further research into risk factors for TVR elucidated that age ≥75, acute coronary syndrome presentation and distal/bifurcation left main (LM) lesion were independently associated with TVR rates.13 TVR rates for ostial/shaft ULMCA PCI and single-stent distal LM disease have been reported to be <5 %. Two-stent technique for bifurcation lesions has been shown to confer higher TVR rates up to 25 % and the optimal technique (minicrush, T-stent, etc.) remains unknown. Nonetheless, it was expected that advances in stent design and introduction of newer DES would impact TVR rates based on results of meta-analyses showing progressive reduction in TVR rates with newer generation DES.14,15
Given the growing body of evidence supporting the non-inferiority of PCI compared to CABG in regard to mortality, guidelines from the American Heart Association and the European Society of Cardiology, which listed PCI for ULMCA as a Class III indication, were updated in 2011.16 PCI for ULMCA disease was assigned a Class IIb recommendation for patients with low to intermediate (≤32) SYNTAX score and bifurcation ULMCA disease, and a Class IIa recommendation for those with low SYNTAX scores (<23) and ostial/shaft ULMCA disease. However, the evidence supporting these recommendations was considered to be imperfect given it was derived primarily from the ULMCA subgroup analysis of the SYNTAX trial, which was considered to be hypothesis generating only. Furthermore, the effect of latest generation DES on MI and TVR rates was not represented in any randomised clinical trial in this patient population. The Nordic–Baltic– British Left Main Revascularization (NOBLE) study and the Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization (EXCEL) trials were designed to address these shortcomings in the available evidence.17,18
The NOBLE trial was designed as a prospective, open-label noninferiority trial and successfully randomised 1201 patients in 36 centres in northern Europe in a 1:1 fashion to PCI or CABG. Five hundred and ninety-eight patients were assigned to PCI group with the majority (92 %) of patients receiving BIOLIMUS™-eluting stents (Biosensors International Ltd.). Ostial and midshaft lesions were treated with single-stent technique while bifurcation/distal LM lesions could be treated with the two-stent technique. Intravascular ultrasound was strongly recommended pre- and post-stent deployment. Six hundred and three patients were assigned to the CABG group and current standard of care practices were recommended, including left internal mammary grafting for revascularisation of the left anterior descending artery and saphenous vein or arterial grafts for other vessel revascularisation. The primary endpoint was a composite of MACCE (death from any cause, non-procedural MI, repeat revascularisation or stroke). Secondary endpoints included the individual components of the primary endpoint, definite stent thrombosis and symptomatic graft occlusion. In the PCI group, 580 of 595 patients underwent PCI and seven received CABG instead. Bifurcation stenting was performed in 88 % of patients, with two-stent technique in 35 % of those patients. In the CABG group, 567 of 592 received CABG and 23 crossed over to PCI arm. Follow-up was available for 90 % of randomised patients at 1 year; however, dropout rates were high with only 38 % and 35 % of patients with follow-up at 5 years in PCI and CABG groups, respectively
The primary outcome occurred in 28 % versus 15 % of patients in PCI versus CABG groups at 5 years (HR 1.51; 95 % CI [1.13–2.0]), which exceeded the prespecified non-inferiority margin (see Table 1). However, at 1 year no difference was noted between the two groups. Analysing the individual components of MACCE, at 5 years no differences were appreciated between the two groups in all-cause mortality or stroke. Repeat revascularisation rates were higher in PCI versus CABG group (15 % versus 10 %, p=0.03) as were non-procedural MIs (6 % versus 2 %, p=0.004). Contrary to prior studies, the SYNTAX score was not associated with outcomes in the PCI group and CABG was favored over PCI in the low SYNTAX score group. At 30 days PCI was favorable to CABG in regard to re-operation for bleeding, transfusions and index hospitalisations. Both the increased rate of non-procedural MIs and the surprising finding that CABG was superior to PCI for low complexity coronary lesions can be explained by the high percentage of distal LM interventions (88 %) in the NOBLE trial. The 8 % BMS use in the PCI group along with higher strut thickness of BIOLIMUS-eluting stents (120 μm) compared to modern everolimus-eluting stents could have impacted the revascularisation, non-procedural MI and stent thrombosis rates given their association with increased thrombogenicity. From a trial design standpoint, critics of the NOBLE trial highlight the change in assessment of MACCE from 2 years to 5 years and ultimately 3 years due to low event rates as a major limitation of this study, which may have biased results. It is also important to note that most randomised trials of ULMCA management, including NOBLE, enrolled CABG patients who are at low surgical risk and, therefore, short- and long-term surgical outcomes may be better in these trials than what can be expected in routine clinical practice.19 Lastly, the generalizability of the results has to be considered given that BIOLIMUS-eluting stents are not currently available in the many non- European countries, including the US.
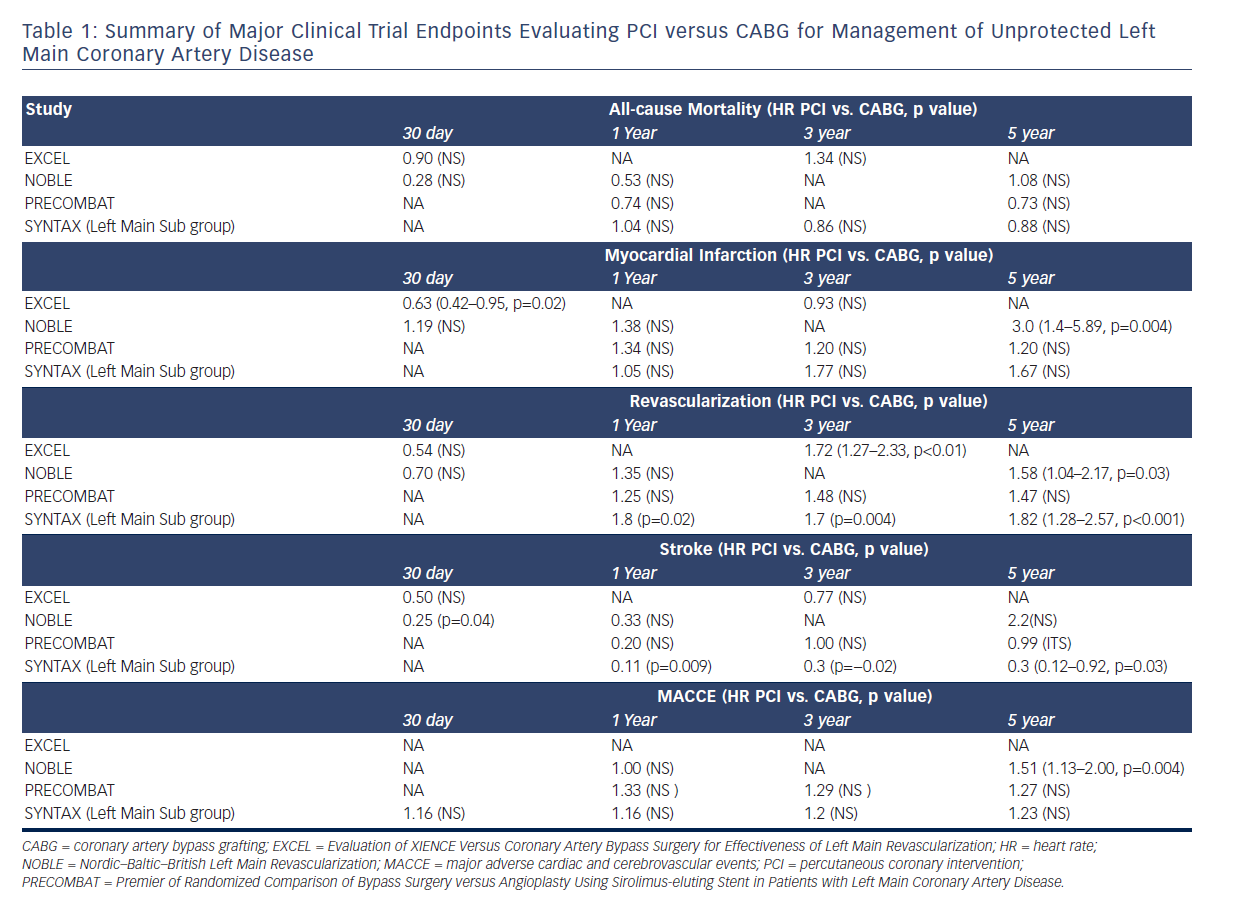
The EXCEL trial was undertaken to specifically test the hypothesis from the SYNTAX ULMCA subgroup analysis that patients with low or intermediate (≤32) SYNTAX scores randomised to PCI or CABG had equivocal outcomes. EXCEL was designed as an international, open-label, multicentre trial that randomised 1905 patients with unprotected ULMCA disease to PCI with XIENCE™ stents (Abbott Laboratories) versus CABG. In the PCI arm, unlike previous trials, complete revascularisation of all ischaemic territories was the expected standard and utilisation of intravascular ultrasound to assess lesion size and stent apposition was strongly recommended. Medical therapy in this arm also reflected current standard of care with dual antiplatelet therapy use mandatory and heparin or bivalirudin use during catheterisation. The management of CABG also reflected current standard of care practices including complete revascularisation, utilisation of arterial grafts for left anterior descending artery revascularisation and utilisation of appropriate antiplatelet therapy in the perioperative and postoperative periods. The primary outcome in the trial was a composite endpoint of death, stroke or MI at 3 years. Secondary outcomes included composite endpoint of all-cause mortality, stroke, MI or ischaemia-driven revascularisation at 3 years, stroke at 30 days, ischaemia-driven revascularisation at 3 years or health-related quality of life and treatment costs.
Of the 1905 patients randomised in the trial, 942 of 948 in the PCI arm underwent complete revascularisation (mean 2.4 stents/patient) and 940 of 957 in the CABG group underwent complete surgical revascularisation (mean 2.6 grafts/patient). The mean duration of follow-up was 3 years in both groups. The primary endpoint occurred in 15.4 % of patients in PCI group versus 14.7 % of patients in CABG group at 3 years (HR 1.0; 95 % CI [0.79–1.26], p=0.02 for inferiority). However at 30 days PCI was favored for the same composite endpoint (HR 0.61; 95 % CI [0.42–0.88], p=0.008), driven primarily by fewer MIs in the PCI arm, with a late catch-up noted by landmark analysis in the PCI group. Consistent with previous studies, within 30 days of revascularisation PCI had fewer adverse events including arrhythmias, infections requiring antibiotics and blood transfusions, compared to CABG (8.1 % versus 23 %, p<0.001). However, ischaemia-driven revascularisation remained higher in the PCI group (12.6 % versus 7.5 %, p<0.001), though stent thrombosis rates with XIENCE stents were lower (0.7 %) than the incidence of symptomatic graft occlusion as well as the stent thrombosis rate observed in the SYNTAX trial with TAXUS stents, highlighting the reduced thrombogenicity of the newer generation DES.
There are several important takeaway points from the EXCEL trial that must be highlighted. The EXCEL trial confirmed that PCI appears to be equivalent to CABG in regard to meaningful outcomes of mortality, stroke and MI at 3-year follow-up in patients with low or intermediate risk anatomy with SYNTAX score ≤32. It is important to note that the primary endpoints for the EXCEL and NOBLE trials were different: the NOBLE trial included repeat revascularisation in the composite primary endpoint, with increased revascularisation partly driving the difference between CABG and PCI groups in the primary endpoint; the SYNTAX trial demonstrated that increased revascularisation does not translate to increased rates of MI or death and so the validity of this primary outcome design has been questioned. Furthermore, while critics of this trial cite the apparent separation of the mortality curves at the 3-year follow-up mark, the significance of this trend is unknown based on available data and long-term follow-up at 5 years and beyond is needed to further explore this finding. In this lower-risk population PCI also had less morbidity in the periprocedural period. The importance of this finding cannot be overemphasised as it is likely to affect decision making by patients in regard to personalised treatment plans. Unlike the SYNTAX trial, the EXCEL trial reflects outcomes of PCI and CABG utilising the latest technology and standard of care medical therapies, making the results very applicable to current global practices given the wide scale availability of XIENCE stents.
Conclusion
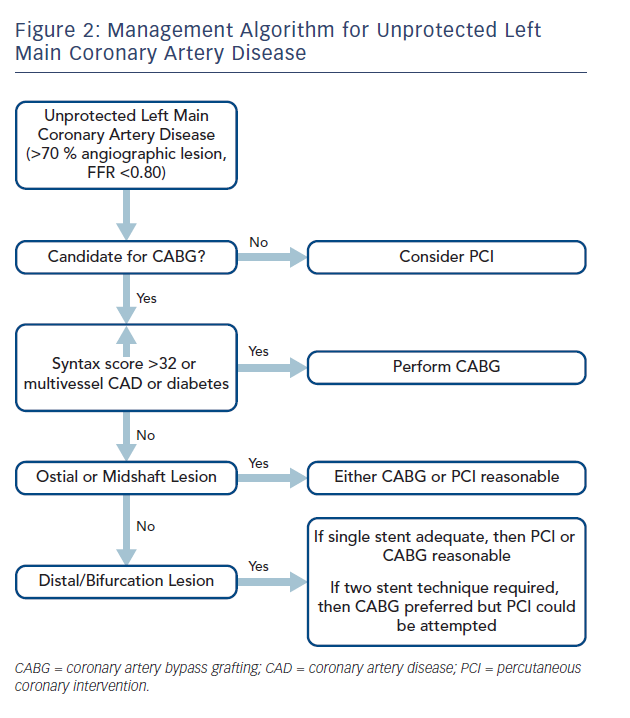
Taken together, the current body of evidence for the optimal management of ULMCA disease does not strongly support the use of either PCI or CABG exclusively for all patients (see Figure 2). Most importantly, there is no mortality difference between the two treatment strategies and a recent meta-analysis including the SYNTAX, PRECOMBAT, Buodriot et al., NOBLE and EXCEL trials shows no difference in safety endpoints.20 In patients with high-risk anatomy or multivessel coronary disease with left main stenosis, CABG is clearly the better management strategy with superior long-term outcomes. For patients with low or intermediate risk anatomy as defined by a SYNTAX score ≤32, either PCI or CABG are reasonable with PCI being associated with less morbidity, shorter hospital stays and lower stroke rates in the periprocedural period than CABG, but also resulting in high rates of repeat revascularisation over time despite use of latest generation DES, procedural techniques and medical therapy. Results of very long-term follow-up of patients in these trials would be particularly useful to assess delayed benefits or shortcomings of either management strategy. Over time it is likely that advances in PCI techniques, including development and adoption of drug-eluting dedicated bifurcation stents for distal LM lesions could reduce revascularisation rates, thereby addressing the Achilles heel of this management strategy. At present, incorporating the results of the NOBLE and EXCEL trials, a personalised, patient-centred approach, should be adopted in the management of ULMCA disease. It is unlikely that additional large randomised trials will be performed comparing CABG and PCI for the management of ULMCA disease in the near future. Given the lack of mortality difference between these two management strategies, the focus should now shift to improving outcomes for both CABG and PCI in ULMCA management.








