MI with non-obstructive coronary arteries (MINOCA) is a common clinical entity, with a prevalence estimated at between 5% and 7% of MI cases, although it has been reported to be as high as 15%.1,2 Compared with patients who have MI with obstructive coronary artery disease (CAD), patients with MINOCA are more likely to be younger, female and non-diabetic, to have lower admission and peak troponin concentrations and to present with non-ST segment elevation MI (NSTEMI).1,3
Patients with MINOCA have a better prognosis than patients with MI and obstructive CAD. However, MINOCA is not a benign condition and MINOCA patients have worse clinical outcomes than matched patients without CAD.1,3,4 Thus, current guidelines recommend that the treating physician should follow a diagnostic work-up seeking the underlying cause of MINOCA.5 Identification of the responsible pathophysiological mechanism is imperative because the various clinical disorders that are potentially the cause of MINOCA have different prognoses and therapies.
Optical coherence tomography (OCT) is an intravascular imaging modality that, because of its high spatial resolution (10–20 μm), can visualise intraluminal and coronary vessel wall microstructures in detail.6 OCT has been shown to be superior to other imaging modalities in identifying, at a coronary level, MI pathologies such as plaque rupture, erosion and intracoronary thrombus.7,8 Therefore, OCT has been proposed as an important tool for the detection of culprit lesions and the assessment of ambiguous or inconclusive angiographic lesions.9
In this review, we describe the use of OCT in delineating causes of MINOCA, review the relevant literature and discuss the therapeutic implications of OCT.
Contemporary Definition and Causes of MINOCA
In the past, the term ‘MINOCA’ has been broadly used, occasionally leading to the misclassification of cases; however, recent European and American scientific documents provide a formal and updated definition of MINOCA.5,10 Accordingly, MINOCA is defined as the fulfilment of the following criteria: acute MI (AMI) as defined by the Fourth Universal Definition of Myocardial Infarction; non-obstructive coronary arteries on coronary angiography, defined as no lesions ≥50% in a major epicardial vessel; and no specific alternative diagnosis for the clinical presentation, such as non-cardiac conditions (i.e. sepsis, pulmonary embolism) or non-ischaemic causes (i.e. myocarditis, takotsubo syndrome and other cardiomyopathies).5,10,11
The above definition is clinically relevant because it differentiates true MINOCA from numerous conditions where a coronary angiogram was performed showing non-obstructive disease. However, MINOCA is still an umbrella term, rather than a definite diagnosis, under which multiple clinical disorders with diverse pathophysiological mechanisms fall. The most recent scientific statement by the American Heart Association discriminates specific aetiologies of MINOCA into ‘atherosclerotic’ and ‘non-atherosclerotic’ causes of myocardial necrosis.10 Plaque disruption is the ‘atherosclerotic’ cause, encompassing its pathoanatomical substrates of plaque rupture, plaque erosion and calcific nodule.12 Non-atherosclerotic causes include epicardial coronary vasospasm, spontaneous coronary artery dissection (SCAD), coronary embolism/thrombosis, microvascular dysfunction and supply–demand mismatch (type 2 MI).10
OCT Delineation of Specific Causes of MINOCA
Atherosclerotic Causes
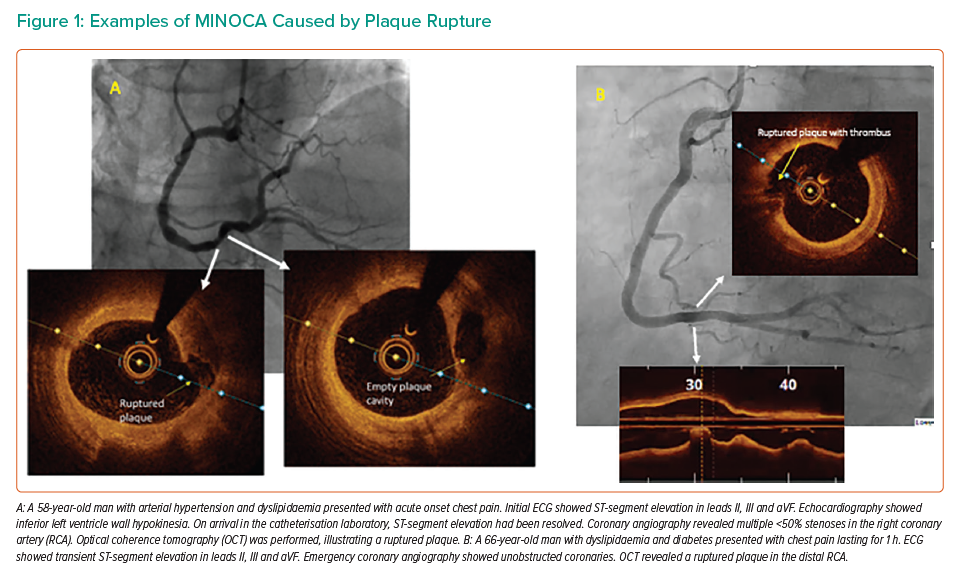
Plaque rupture is defined as a discontinuity of the fibrous cap of thin cap fibroatheroma with underlying lipid and a necrotic core, with or without core ‘washout’ or associated thrombus.6 Examples of plaque rupture in patients with MINOCA are shown in Figure 1.
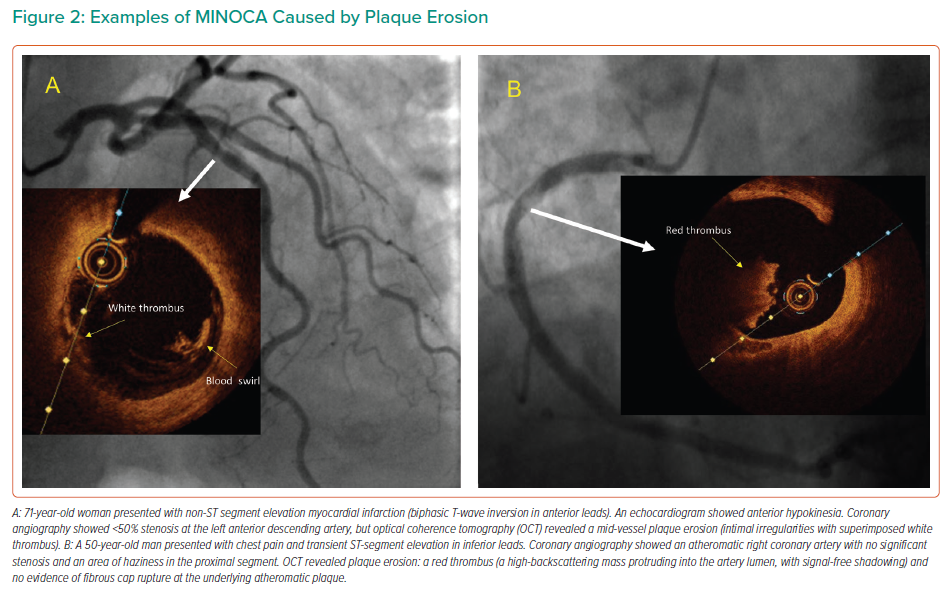
Plaque erosion is characterised by the presence of thrombi and an irregular luminal surface with no evidence of plaque rupture.6 Examples of plaque erosion in patients with MINOCA are shown in Figure 2.
Calcified nodule is defined as one or more protruding, signal-poor and well-delineated regions (characteristics that imply the presence of calcium) frequently forming sharp, jutting angles.6 In contrast to plaque erosion, which is more prevalent in young, premenopausal women, calcified nodules are found in older, diabetic patients.10
Non-atherosclerotic Causes of MINOCA
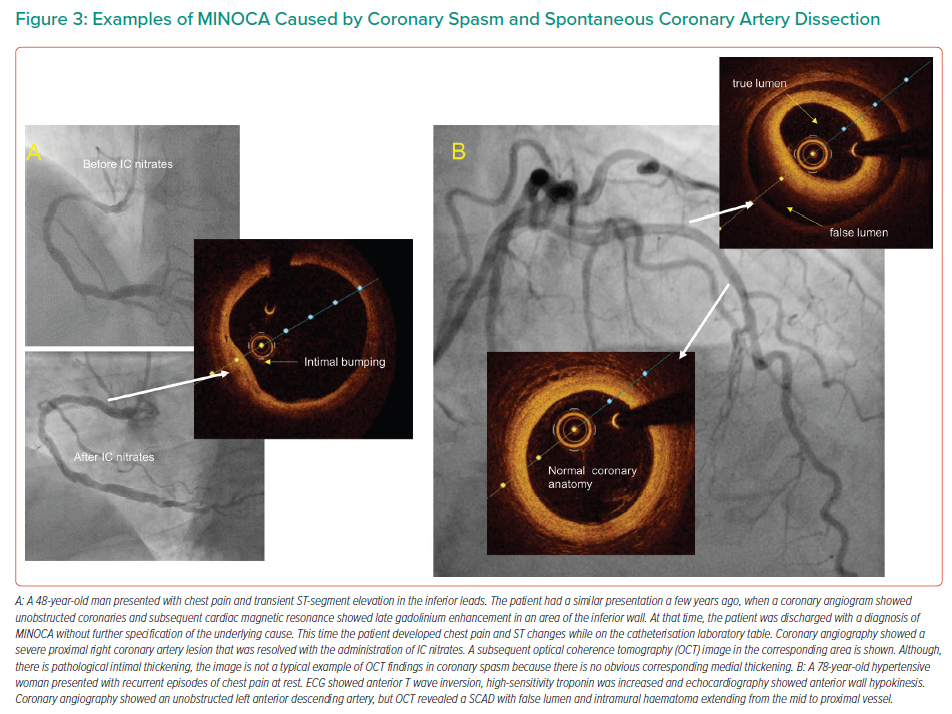
Coronary artery spasm is a recognised cause of MINOCA.10 Angiographic resolution of coronary spasm by nitrates could provide the diagnosis, but often MINOCA patients undergo angiography remote from the acute phase. Provocative testing for coronary artery spasm is the test of choice when vasomotor abnormalities are suspected.13 OCT can identify areas of intimal bumping (intimal projections into the lumen with thickening of the media), which have been found to correspond to areas of spasm.14 An example of a coronary spasm as a cause of MINOCA is shown in Figure 3.
SCAD is a spontaneous separation of the coronary artery wall that is not iatrogenic, and not related to trauma or atherosclerosis.15 SCAD angiographic characteristics vary, and a standardised classification has been proposed.9 Type 1 SCAD represents the classical linear coronary defect with potential arterial wall stain. Type 2 SCAD is characterised by an abrupt reduction in vessel size and subsequent normalisation or with persistent size reduction to the distal vessel and is the most observed type. SCAD can also mimic atherosclerosis (Type 3) or simply present with abrupt vessel closure (Type 4). OCT can delineate the diagnosis when there is angiographic uncertainty (typically Types 3 and 4) because it produces characteristic images of SCAD.9 Nevertheless, it should only be used when deemed safe and necessary for diagnostic purposes because vessel manipulation and contrast injection could propagate the dissection plane.16 An example of SCAD as a cause of MINOCA is shown in Figure 4.
Existing Evidence of OCT Use in MINOCA
A small number of studies examined the usefulness of OCT in MINOCA patients (Table 1). The design of these studies varied widely with regard to the studied population, the description and classification of OCT findings and the diagnostic work-up followed. For example, the provocation test was used to exclude epicardial spasm as the cause of MINOCA before performing OCT in one study.17 In another study, OCT was encouraged in all three vessels regardless of the ECG, echocardiography and coronary angiography findings.18 Some studies used OCT with cardiac magnetic resonance (CMR) to evaluate the diagnostic yield of this combination, as well as the agreement of these two imaging modalities regarding the final diagnosis.17–19 Some of these studies are heavily limited by the small number of patients and their single-centre design.
In a retrospective single-centre study, Yamamoto et al. sought to evaluate the morphological characteristics of non-obstructive coronary lesions as assessed by OCT in a mixed population of acute coronary syndrome (ACS) and stable CAD patients.20 In this small ACS cohort (n=31), the incidence of any ‘high-risk OCT finding’ (i.e. plaque rupture, calcified nodule, intimal laceration or thrombus) was 25.8%, with thrombus being recognised in 12.9% of cases.20 Interestingly, the rate of ‘high-risk findings’ was similar in the stable CAD group (22.5%; p=0.70).20
Another small, single-centre study reported OCT findings in patients with ACS and non-significant coronary lesions on coronary angiography.21 In that study, an ‘unstable plaque’ was found in 78% of patients. Specifically, plaque erosion (41%) was the most common finding, followed by plaque rupture (30%) and calcified nodule with thrombus/plaque disruption (7%).21 Although all patients had angiographic coronary stenoses of <50%, percutaneous coronary intervention (PCI) was performed in 95% of those with an unstable plaque identified by OCT.21 The value of that study is questionable due to the very high rate of revascularisation in lesions causing mild stenosis, a practice that, as discussed below in the Therapeutic Implications section, is not generally recommended.9,10
Opolski et al. were the first investigators to follow a robust prospective methodology including the combination of OCT with CMR in the work-up of MINOCA patients. In their study, they included 38 patients with MI and coronary stenosis of ≤50%.19 Plaque rupture, plaque erosion and calcified nodule were identified in 8 (21%), 4 (11%) and 2 (5%) patients respectively, with the total percentage of patients with an underlying atherosclerotic mechanism of MINOCA being 36% (one patient had both plaque rupture and calcified nodule).19 Interestingly, immediate interpretation of OCT led to a change in therapeutic plan in 16% of cases, including referral for PCI in five patients and/or modification of antithrombotic therapy for two patients.19 In a subgroup of 31 patients who underwent CMR, ischaemic-type late gadolinium enhancement (LGE) was present in 7 (23%) and was more common in patients with than without plaque disruption (50% versus 13%, respectively; p=0.053) and coronary thrombus (67% versus 12%, respectively; p=0.014).19
In their study, Taruya et al. included 82 consecutive patients with ACS and non-obstructive CAD who underwent OCT and had clinical follow up for up to 2 years.22 High-risk lesions in the culprit artery were identified in approximately half the patients (51.2%), including ruptured plaque (15.9%), calcified nodule (11.0%), SCAD (8.5%), lone thrombus (8.5%) and plaque erosion (1.2%).22 Although none of the patients without high-risk lesions experienced major adverse cardiovascular events (MACE), four (10%) of the patients in the high-risk-lesion group had a recurrent ACS event with obstructive coronary artery stenosis. All the recurrent ACS events occurred at the segment where the high-risk lesion had been originally identified.22
Gerbaud et al. evaluated the diagnostic yield of OCT accompanied by CMR in a highly selected group of 40 MINOCA patients.17 The authors followed the contemporary MINOCA definition, and their cohort was carefully selected because they only included patients with a suspected diagnosis of epicardial cause based on the correlation between ECG changes and regional wall motion abnormalities (WMA) observed either on admission echocardiography or left ventricle angiogram. Furthermore, before final inclusion, the authors used provocation testing for coronary artery spasm in patients presenting with suspected vasospastic angina according to COVADIS (Coronary Vasomotion Disorders International Study) group recommendations.23 In the final study cohort, OCT identified a pathological substrate in 80% of cases.17 Plaque rupture (35%) and plaque erosion (30%) were the most commonly recognised potential MINOCA mechanisms, followed by lone thrombus (7.5%), SCAD (5%) and eruptive calcific nodule (2.5%). OCT findings changed medical management in 11 patients (27.5%). AMI was evident at CMR in 31 of 40 patients (77.5%). Twenty-three patients (57.5%) had a substrate and/or diagnosis supported by both techniques, with an evident relationship between the findings obtained by the two techniques. By coupling OCT with CMR, a substrate and/or diagnosis was found in 100% of cases.17
Reynolds et al. conducted the largest study to date in the field, including 145 women with MINOCA who per protocol would undergo multivessel (ideally three-vessel) OCT followed by CMR within 1 week.18 Eventually, OCT in all three major coronary arteries was performed in 59.3% of cases. A definite or possible culprit lesion was identified on OCT in 67 (46.2%) of patients, with the most common culprit lesions being intraplaque cavity (21.4%), layered plaque (13.1%), plaque rupture (5.5%) and thrombus without plaque rupture (3.1%; i.e. thrombus overlying an intact fibrous cap, or lone thrombus). Furthermore, three patients (2.1%) had intimal bumping suggesting coronary artery spasm and one (0.7%) had SCAD.18 Not surprisingly, there was no calcified nodule identified, a phenotype that is more common among older, male, diabetic patients. CMR, which was available in 116 of the 145 participants, was abnormal in 74.1% of cases with an ischaemic pattern of LGE, regional injury or non-ischaemic findings (i.e. myocarditis, takotsubo and other cardiomyopathies) in 32.8%, 20.7% and 20.7% of cases, respectively.18 After the combination of OCT and CMR, a cause was identified in 84.5% of women, with an ischaemic cause/MI being confirmed in 63.8% of cases.18
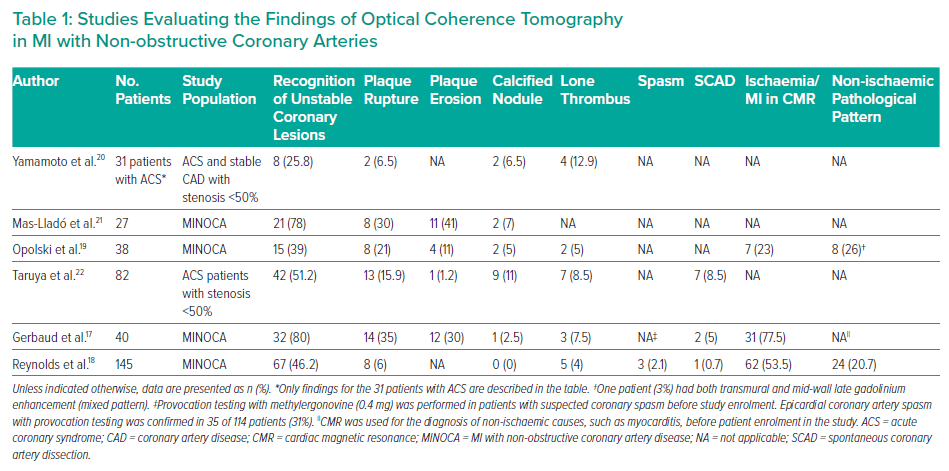
Although Gerbaud et al. identified abnormal findings on OCT in 80% of patients, Reynolds et al. identified a culprit lesion by OCT in less than half of the patients (46%).17,18 This discrepancy is not surprising if the screening/recruiting process of the two studies is considered. Gerbaud et al. followed the contemporary MINOCA definition and mandated ECG changes and correlated WMA for all included cases.10,17 In contrast, in the study of Reynolds et al., 35% of patients had a normal ECG and only 44.1% had WMA on echocardiography.18 The latter study was conducted exclusively in women; hence, the results cannot be extrapolated to men. In addition, the study was criticised because it included layered plaque among the unstable plaque phenotypes. The formation of such a plaque may take weeks to months and likely represents a sequela rather than a pathophysiological mechanism of ACS.24
Therapeutic Implications
Robust scientific data with respect to MINOCA therapeutic strategies are missing. In any case, the diverse population with multiple discrete underlying pathologies that is described under the umbrella term of MINOCA would make a one-size-fits-all strategy faulty and meaningless. This is probably the disadvantage of a large observational study derived by the SWEDEHART registry, which reported that dual antiplatelet therapy (DAPT) showed no benefit.25 Because multiple distinct pathologies could be involved in the clinical presentation of MINOCA, it seems intuitive that subsequent management should be based on the final established underlying cause. This is supported by the current European guidelines, which recommend the management of patients with an initial diagnosis of MINOCA and a final established underlying cause according to disease-specific guidelines.5 In cases in which a final diagnosis is not reached, the guidelines state that secondary prevention for atherosclerotic disease may be applied.5
When OCT has demonstrated plaque disruption (rupture, erosion or calcific nodule) as the underlying cause, the administration of a P2Y12 receptor inhibitor in addition to aspirin seems logical based on studies of patients with MI that did not discriminate between obstructive and non-obstructive CAD.26 The duration of DAPT should be individualised after taking into consideration the bleeding risk as well as the therapeutic management (medical versus invasive) of the patient with the by-default strategy being DAPT administration for 12 months followed by lifetime single antiplatelet therapy.27
Regarding the need for routine stent implantation, more research is needed because currently there is no consensus and management remains controversial. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions is in favour of PCI when intravascular imaging identifies a ruptured plaque in MINOCA patients.9 According to the same statement, stenting can be deferred in cases of plaque erosion when the lesion is non-obstructive and flow has been restored.9 In contrast, a scientific statement from the American Heart Association recommends against routine stenting in cases of plaque rupture and erosion with no residual significant stenosis without discriminating between the two mechanisms.10
A small randomised trial (EROSION study) on patients with ACS caused by plaque erosion that leads to ≤70% stenosis demonstrated that DAPT with aspirin and ticagrelor without stenting was associated with favourable outcomes at 1 year, with approximately 93% of patients remaining without MACE, supporting the concept of medical-only management in this group of patients.28 There are no studies or expert recommendations when the underlying lesion is an eruptive calcific nodule. What should be considered though is the fact that calcified nodules are related to stent underexpansion and higher rates of future target vessel revascularisation.29 Therefore, when PCI is deemed necessary, it should be accompanied by calcium modification techniques.
When the cause of MINOCA is one of the non-atherosclerotic causes, management should be according to the underlying condition. For example, when vasospasm is identified as the underling pathophysiological mechanism, β-blockers should be halted/avoided and the patient should be started on calcium channel blockers, which are the agents of choice with short-acting nitrates being the second option.10,27 Regarding SCAD, there are no randomised controlled trials to guide management, and the use and duration of aspirin alone or DAPT in medically managed cases remains controversial. A recent document by a panel of experts suggested DAPT for 2–4 months followed by low-dose aspirin as monotherapy for up to 12 months; aspirin alone or not antiplatelet therapy at all was proposed as a reasonable alternative for patients at high bleeding risk.15 The same document emphasised that patient counselling and shared decision making should be employed, especially among the younger, female population, which carries an advanced risk of bleeding (e.g. due to menorrhagia).15 Importantly, a recently published multicentre SCAD registry that investigated single and dual antiplatelet regimens on conservatively treated patients challenged the paradigm of DAPT in SCAD.30 That study showed that prolonged DAPT (97% of patients received DAPT for 12 months) was independently associated with a higher rate of adverse cardiovascular events at the 1-year follow-up.30
Regarding further intervention, spontaneous angiographic healing is the natural history of the disease in 95% of patients, highlighting that conservative therapy is preferred in stable patients without ongoing ischaemia, as in cases with MINOCA where, by definition, there is no significant angiographic coronary stenosis.31 In any case, it should be highlighted that intervention in SCAD cases carries elevated risks.32
Clinical Perspectives
Current scientific documents underline the significance of CMR in MINOCA. European guidelines for the management of ACS without persistent ST-segment elevation recommend the performance of CMR in all MINOCA patients without an obvious underlying cause (class I, level of evidence B).5 The guidelines do not give any specific recommendation for the utilisation of OCT, although the recent studies that support the use of CMR (e.g. Reynolds et al.) have shown the value of OCT too.17–19 CMR is an excellent modality to discriminate between ischaemic and non-ischaemic causes of MINOCA, as discussed previously. However, CMR cannot inform us regarding the underlying pathology of the ischaemic insult: for example, it cannot tell us whether the ischaemia was due to a ruptured plaque or coronary spasm. As shown previously, OCT can discriminate the various phenotypes of epicardial pathologies.9,14,16–22 Importantly, it can take place at the time of coronary angiography, guiding immediate management. Conversely, ‘blind’ three-vessel OCT is not practical and the diagnostic yield of such a strategy is low.
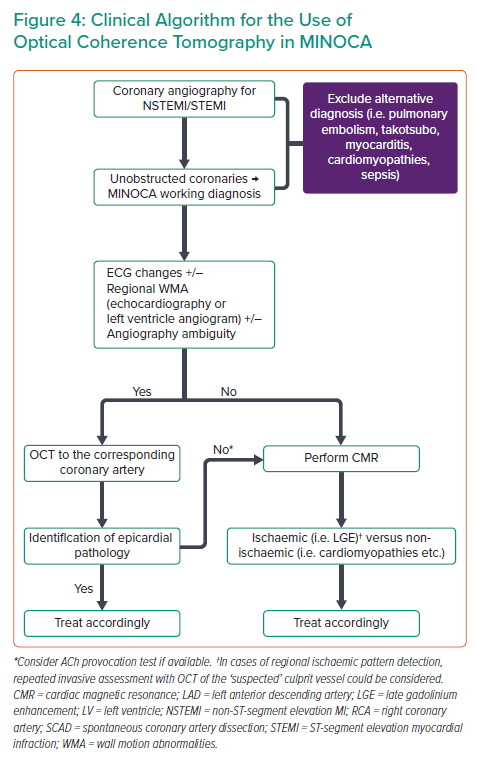
It is characteristic that in the study of Reynolds et al., although three-vessel OCT was recommended, it took place in 59.3% of cases, highlighting the practical limitations of such a strategy.18 In addition, it is likely that ‘blind’ three-vessel OCT, not guided by electrocardiographic and imaging findings, could lead to the erroneous recognition of a bystander lesion as the culprit because ‘unstable’ plaques have been also found in patients with stable CAD.20 Furthermore, as discussed previously, the rate of pathological findings is much lower when compared with OCT guided by ECG and echocardiography/left ventricle angiogram findings. Considering the above aspects, we propose a practical algorithm for OCT use in cases of MINOCA (Figure 4). In our algorithm, OCT vessel interrogation is guided by ECG changes and regional WMA, and is suggested in the corresponding coronary artery. A hazy non-obstructive coronary lesion in a MINOCA case is highly suspicious of a coronary pathology and should prompt further investigation by OCT. However, even when the vessel appears smooth in angiography, there still may be an underlying pathology (i.e. Figure 1B) and OCT should be considered in the artery corresponding to ECG changes and/or WMA.
Finally, it needs to be acknowledged that even in coronary causes of MINOCA, OCT cannot always give a definite diagnosis. OCT could generate the suspicion for the presence of epicardial coronary spasm, but it is not the test of choice. Furthermore, OCT does not provide any information regarding coronary microvascular spasm, which plays a role in several MINOCA cases.13 In patients with suspected coronary vasomotor abnormalities, a provocation test with acetylcholine should be considered to assess the presence of epicardial or microvascular spasm33 (Figure 4). Provocation testing in MINOCA patients has been shown to be safe and able to identify high-risk patients.13 However, its use in clinical practice is currently limited and mainly restricted to specialised centers.34 Thrombus embolism not related to plaque disruption is another coronary cause of MINOCA where OCT is not diagnostic. A patient’s clinical history and characteristics could set the suspicion of embolism (e.g. prosthetic heart valves, apical thrombus, infective endocarditis or myxoma) and echocardiography could be useful in delineating the cause of MINOCA in such cases.33
Conclusion
OCT is an indispensable tool for the recognition of the underling pathogenetic mechanism of MINOCA when epicardial pathology is suspected because it can reliably identify pathologies not apparent on coronary angiography. OCT should be part of the diagnostic work-up for a patient with MINOCA when ECG changes and WMA on an echocardiogram or left ventricle angiogram indicate a localised epicardial coronary cause.