Radial vs Femoral Access
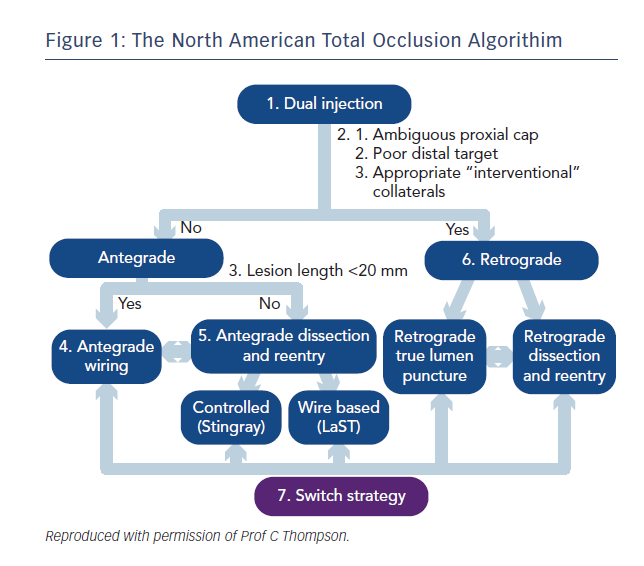
In recent years several large registries and randomised controlled trials have demonstrated a mortality benefit for trans-radial over trans-femoral percutaneous coronary intervention (PCI), likely mediated by reduced bleeding complications.1–4 Building on the pioneering Japanese experience, the advent of the hybrid algorithim approach, coupled with innovative new technologies has lead to a global renewed focus and enthusiasm for chronic total occlusion (CTO) PCI with marked increase in procedural success rates.5 This approach combines the skill sets of antegrade wire escalation (AWE), antegrade dissection re-entry (ADR), retrograde wire escalation and retrograde dissection re-entry (RDR) with keen attention to minimising procedure time, contrast volume and radiation exposure (see Figure 1).
Arterial Access in CTO PCI
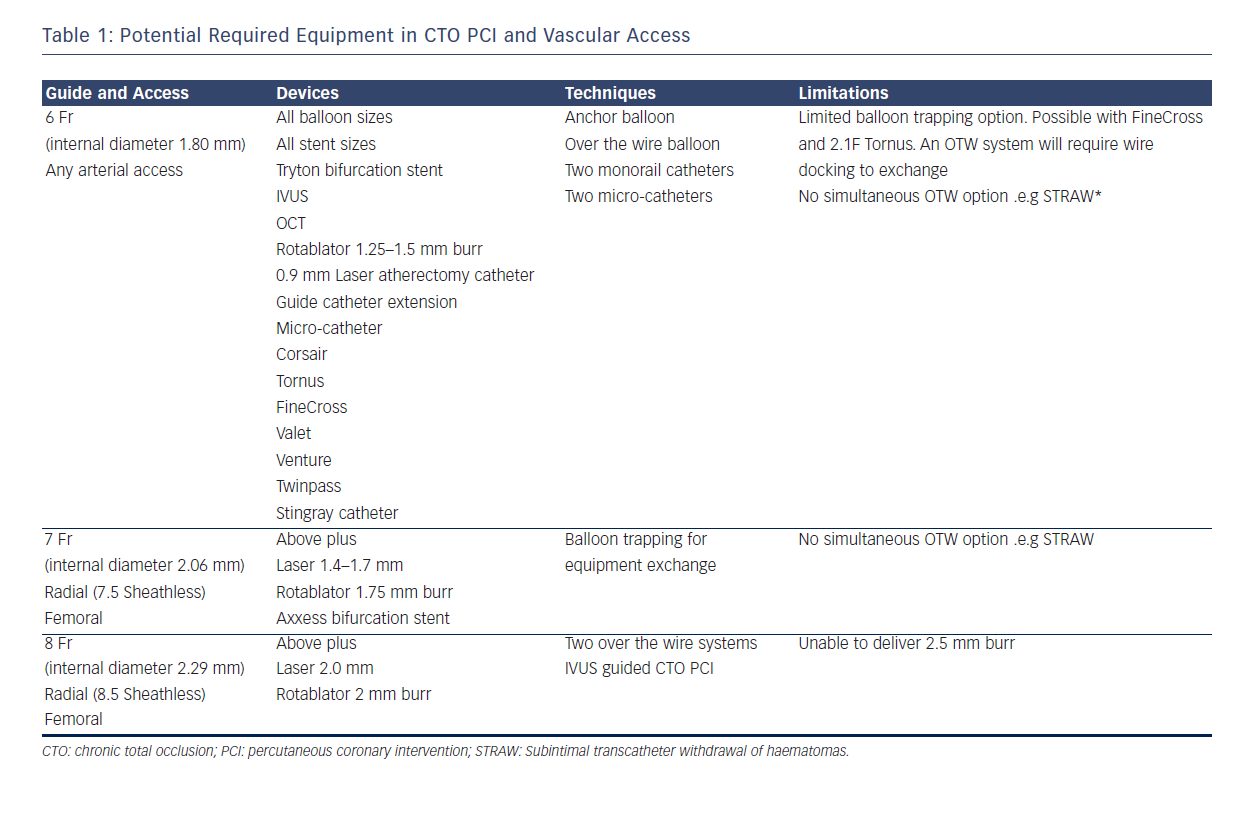
One challenge, however, in CTO PCI remains the risks of multiple and larger vascular access. Whilst for conventional PCI the direction of travel is towards miniaturisation with smaller French sizes and sheathless approaches,6 in CTO PCI, larger French systems are required to offer capability across the full spectrum of techniques (see Table 1). In addition, CTO procedures are often dependent on visualisation of the occluded vessel by contra-lateral collaterals requiring dual access sites and contra-lateral coronary injection hence immediate potential to double the risks of access complication. Furthermore the thrombotic risk of equipment in both coronary circulations requires meticulous anticoagulation management with a target activated clotting time (ACT) greater than 300s measured systematically every 20–30 minutes. This contrasts with a target of 250s for conventional single access site non-CTO PCI and might exacerbate any access related bleed.
Table 1 describes the procedural techniques as accommodated by different guide catheter sizes. 8F is required to accommodate two over-the-wire (OTW) technologies and also offers greater passive guide catheter back-up to facilitate penetration of the CTO proximal cap, an essential step and when unsuccessful, a frequent mode of failure. The radial and ulnar arteries infrequently have an inner luminal diameter larger than 2.5 mm in men at the wrist making insertion of a 7F sheath (outer diameter 3.06 mm) a usual maximum in men and 6F sheath (outer diameter 2.67 mm) in women.7,8 The requirement for an 8F system has resulted in CTO PCI bucking the trend towards trans-radial PCI with high use of trans-femoral access. This has implications for training of medical and nursing staff (operative and after care) in increasingly radial dominant centres. Below we discuss the evidence for best practice with respect to femoral puncture technique and also assess the technologies and techniques available to place larger inner diameter catheters into the radial artery.
Femoral Artery Puncture Techniques
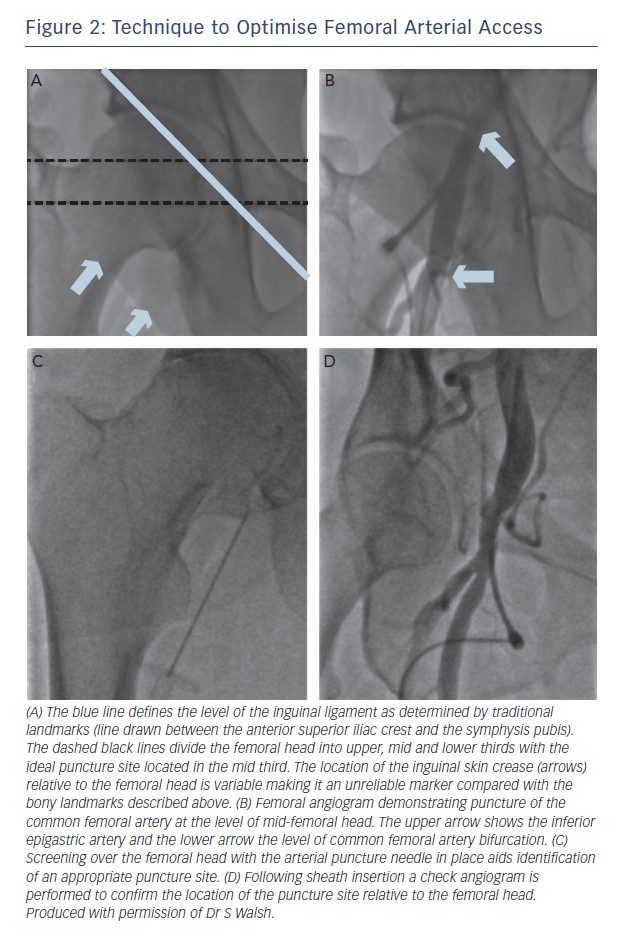
Optimal location of the anatomical puncture site is the most crucial factor in minimising arterial complication rates. Arteriotomy positions below the common femoral artery (CFA) bifurcation or above the origin of the inferior epigastric artery (IEA) are associated with significant vascular complications.9 There is marked variability in inter-individual anatomy and surface landmarks may not reflect optimal puncture position. Traditionally, the inguinal ligament was used as a suitable landmark because it acts as a surrogate marker for the IEA and retroperitoneum.10
The ‘ideal’ anatomical puncture site is over the mid-portion femoral head, above the CFA bifurcation and below the inguinal ligament.11 Pitta et al. cited vascular complication rates of 18 % when punctures were performed outside of these anatomical landmarks.9 In particular, arterial cannulation above the inguinal ligament (and above the inferior epigastric artery) increases the risk of retroperitoneal haemorrhage due to a lack of ability to compress the femoral artery using the femoral head.12There are three commonly used techniques to gain femoral arterial access: anatomical landmarks, fluoroscopy guided and ultrasound guided punctures. Anatomical landmarks including skin crease location, maximal palpated pulse intensity and bony landmarks are not reliable methods.10
Fluoroscopy-guided Femoral Punctures
Fluoroscopic landmarks have been widely used in every day interventional practice and are described in Figure 2. The clinical benefits of this modality have not been reported in randomised trials. However, use of fluoroscopic-guided femoral arterial access has been demonstrated to reduce bleeding-related complications in female patients.13 In addition, prospective registry data have demonstrated fluoroscopy use prior to arterial puncture was associated with a lower incidence of pseudoaneuryms (0.3 % vs. 1.15 %, p=0.017), any arterial injury (0.7 % vs. 1.9 %, p<0.01), and hospitalisation (2.1 days vs. 2.4 days, p<0.01).14 Recent multicentre observational data has also demonstrated an extremely low incidence of vascular-access complications (0.89 %) in CTO PCI cases performed by femoral arterial access (81 % 8F sheaths) using routine fluoroscopic guidance.15
Ultrasound (US) Guided Femoral Arterial Access
The important role of US guided venous cannulation is unquestionable. US guided arterial puncture in interventional cardiology is increasingly being adopted as the method of choice, especially in more complex cases such as CTO PCI. Utilisation of ultrasound-guided femoral access can enable direct visualisation of the CFA bifurcation and identify anterior puncture of the needle through the CFA.11 The success of ultrasound-guided femoral arterial puncture was demonstrated in the Femoral Arterial Access With Ultrasound (FAUST) trial, with a reduced number of puncture attempts, improved first pass success rates, reduced time to successful access and reduced vascular complications.16
Micropuncture Technique
Arterial puncture with a small 21-gauge micropuncture needle with a 0.018-inch guide wire (Cook Medical, Bloomington, USA) theoretically increases the chances of obtaining haemostasis with manual compression if the puncture is in a vein. A single-centre, retrospective study that compared a 21-gauge needle vs. an 18-gauge needle has shown no significant difference in access site complications. Randomised data is therefore needed to determine whether the micropuncture technique can reduce vascular complications.11
Access Site Management
Uncontrolled hypertension has been identified as a risk factor for femoral access complication and optimal blood pressure control should be achieved prior to sheath removal.11 Data on vascular closure devices (VCD) are controversial and have provided mixed results, with early studies reporting increased rates of vascular complications while more contemporary registries have reported less bleeding complications compared to manual haemostasis.11
An appropriately powered randomised trial comparing newer generation VCD (after an appropriate learning curve) and manual haemostasis is needed to determine the preferred strategy for obtaining haemostasis. Minimising sheath dwell time may also improve outcomes. Early arterial sheath removal after PCI has been associated with reduced bleeding complications.17
Tips and Techniques To Use Larger Guides Trans-Radially
Passage of larger guiding catheters up the radial artery is limited by absolute vessel size and arterial spasm. In a recent multicentre registry containing over 1900 transradial procedures the incidence of radial spasm was 2.7 %, with multiple puncture attempts and use of larger introducer sheaths (7F) being independent predictors of radial spasm.18 Intra-arterial vasodilators reduce the incidence of radial spasm although the optimal “spasmolytic” cocktail remains to be identified and their use in some patients may result in hypotension and bradycardia. The use of hydrophilic sheaths and catheters can also further reduce spasm.19
Spasm can also be minimised by gradual graded gentle dilatation by tapered introducers. If resistance is encountered when upsizing from a 5F catheter to a 6F guide and forearm angiography has excluded an anatomical variation such as a radial loop or accessory artery, often balloon assisted tracking is sufficient to allow the new catheter to pass.
In this technique a coronary guidewire is passed into the guide and into the aortic root (alongside the 0.035” wire) and a 2.0 mm monorail balloon inflated two-thirds in and one third exiting the guide to provide a soft rounded shoulder tip.20 In a male patient with a 7F sheath experiencing resistance to 7F guide passage the same technique using a 2.5 mm balloon is often successful. Another technique to provide a more tapered tip to facilitate guide passage is a mother-in-child configuration, so the guide is lead by a smaller diameter section, e.g. use of a 4F Judkins Right 4 (JR4) or multipurpose (MPA) inside a 6F guide (requires either a longer 4F diagnostic >110 cm or shorter 90 cm guide), or similarly a 5F JR4 or MPA inside a 7F guide.
An elegant innovative solution is the use of sheathless guiding catheters. These are commercially available (Asahi Intecc Co Ltd, Aichi) in 6.5F, 7.5F and 8.5F sizes with inner diameters (I.D.) identical to 6F, 7F and 8F guides respectively. The outer diameters (O.D.), however, are less than a standard 5F sheath for 6.5F sheathless, <6F sheath for 7.5F sheathless and <7F sheath for the 8.5F sheathless and can hence be anatomically accommodated in the majority of radial arteries. The same principle is employed by “home-made” sheathless guides using commercially available tapered introducers with standard guide catheters. This has the advantage of free choice of shape and manufacturer of guide. Introducers include the Shuttle (Cook Medical, Bloomington, USA), a carotid device: 5F Shuttle introducer in 7F guide; 6F Shuttle introducer in 8F guide. The use of sheathless guides requires specific expertise as the highly hydrophilic coating may increase catheter instability. An additional drawback is that use of 150 cm devices, such as microcatheters in retrograde cases, may require guide catheters less than 100 cm, especially in taller patients. Sheathless guides are not available in shorter lengths. However, to overcome this, the tapered introducers from the Asahi commercial sheathless guides can be used inside conventional 90 cm guides for the same purpose and when guide exchange is required.
The Second Access Site
Within the hybrid algorhythm the second access site allows contra-lateral coronary injections for visualisation of the target for AWE and ADR techniques and a 6F system will suffice. However adopting a retrograde approach either as a primary strategy or bail-out for failed antegrade approach requires a capability for passage of guidewires, microcatheters and balloons into the donor vessel (giving rise to septal or epicardial collaterals). 6F is a minimum but 7F has advantages of better contrast delivery, better passive support and importantly the ability to use a wider range of equipment and techniques. (see Table 1) It is therefore evident that the selection of 6F guide catheters may profoundly limit some of the potential CTO techniques such that larger diameter guides are needed when more complex techniques are planned or anticipated. Recommendation of radial vs. femoral access for the second arterial site is also difficult due to the fact that no randomised data exists on transradial access in CTO PCI and all of the registry data is single centre or single operator derived and conducted by operators with specific experience in transradial PCI.21 Radial access therefore constitutes a valuable alternative to femoral access in CTO PCI in high volume radial centres, with anticipated equipment and techniques taken into consideration.
Radial vs Femoral Dominance
Promotion of a high volume of radial access may interfere with the equally important goal of maintaining proficiency in the femoral approach, which is indispensable in a variety of procedures. As with the radial approach, outcomes depend on the experience of operators and centres. Rafie et al. reported higher vascular access complication rates (12.5 %) when default radial operators in a single centre undertook PCI cases via the femoral approach.22 As expected, use of femoral access was associated with increased case complexity and larger arterial sheaths and femoral access was performed without fluoroscopic screening. In contrast, a recent multicentre study has demonstrated an extremely low incidence of vascular-access complications (0.89 %) in CTO PCI cases performed by default radial operators when femoral arterial access is performed using routine fluoroscopic guidance.15
Conclusion
When considering arterial access in CTO PCI, a balance is needed between anticipated procedural difficulty, planned CTO strategy and the desire to minimise the risk of vascular access-related complications. The requirement for larger French systems, particularly in more complex cases has resulted in high use of trans-femoral access in CTO PCI. This has implications for training of medical and nursing staff both in terms of both peri-procedural and post-procedural care in increasingly radial access dominant centres. The miniaturisation of angioplasty equipment continues and is expected to increase the feasibility of transradial CTO interventions.
To reduce the risk of femoral access site complications, femoral access should be achieved with fluoroscopic guidance which is readily available in every catheter lab. A strategy of US guided puncture may become more commonplace in the future.