Dr Romagnoli’s work examined the correlation between the extent of myocardial revascularisation and the functional recovery of the left ventricle (LV) in Impella-protected percutaneous coronary intervention (PCI) patients. LV support is often used in complex and high risk indicated procedures/patients (CHIP). These patients generally present with both LV dysfunction and severe coronary artery disease (CAD), as indicated by a high British Cardiovascular Intervention Society myocardial jeopardy score (BCIS-JS). (The BCIS-JS is a simplified scoring system that classifies the extent of CAD by estimating the amount of myocardium at risk based on the location of coronary artery stenoses. The BCIS-JS is similar to the SYNTAX score, which also uses the number of coronary lesions and their complexity, location and functional impact on the vasculature to qualify the myocardium’s condition.1)
In patients with LV dysfunction and severe CAD, the risk of intraprocedural heart failure is the key factor driving the choice of Impella support in both acute and stable patients. Dr Romagnoli emphasised that the goals of using an Impella device are to reduce periprocedural complications and in-hospital mortality, as well as to achieve a more complete revascularisation regardless of anatomical complexity. Although there is clear clinical evidence to support these intraprocedural or in-hospital benefits,2–6 Dr Romagnoli noted the evidence for long-term benefit is still debated. Therefore, his group sought to determine whether the beneficial effects on procedural outcomes in protected PCI are consistent with functional LV ejection fraction (LVEF) improvement and recovery.
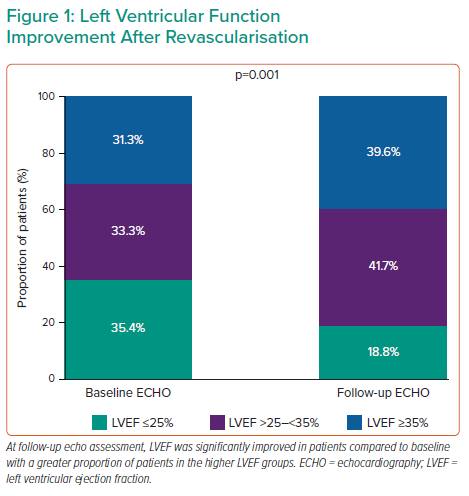
Dr Romagnoli and colleagues designed the ImpEco study, a prospective single-centre registry investigation. The primary goal of the study was to determine whether the acute procedural outcome of Impella-protected PCI in patients with LV dysfunction is associated with substantial LV recovery at follow-up. The study included 48 patients who required mechanical circulatory support during elective or urgent revascularisation. BCIS-JS was collected at the time of angiography, whereas the Wall Motion Index Score (WMSI) and LVEF were collected from transthoracic echocardiography at baseline and within 1 year after the procedure (mean follow-up time: 146 days [42–381]). Dr Romagnoli and colleagues combined the WMSI and BCIS-JS by analysing the changes in WMSI in each ventricular segment according to the segment’s revascularisation status according to the BCIS-JS.Almost all patients (97.9%) had multivessel disease. Both the BCIS-JS and SYNTAX scores were markedly reduced from preprocedural levels, with an overall revascularisation index of 76%. After a median follow-up of 4 months, echocardiographic results revealed no changes in LV end-diastolic and end-systolic diameters, indicating that the patients had not experienced negative remodelling. Conversely, LV end-systolic volume was significantly reduced and LVEF was significantly increased (Figure 1), indicating increased contractility and function. Diastolic function, represented by the E/A ratio, improved as well. The WMSI showed the most significant improvement at follow-up. Interestingly, the improvement was driven by the changes in the revascularised segments, with no change in the non-revascularised segments. The improvement in WMSI was proportional to the dysfunction at baseline and was correlated with the extent of revascularisation.
Dr Romagnoli concluded that in patients with severe CAD and LV dysfunction, Impella support allowed for more extensive revascularisation. More complete revascularisation was associated with mid-term LV contractile recovery, in terms of both systolic and diastolic function. In addition, regional wall motion recovery was more evident in revascularised segments and was correlated with preprocedural dysfunction, which suggests the possibility of a multimodal imaging approach to optimise and personalise revascularisation strategies in patients with such complex presentations.