Coronary chronic total occlusions (CTO) are frequently identified during coronary angiography and remain the most challenging subset of coronary artery lesions to treat. In recent years, the success rate for CTO percutaneous coronary intervention (PCI) has substantially improved up to >90%, thanks to advances in techniques and technologies along with increased operator experience.1–7 Several algorithms have been developed to standardise crossing strategies with the aim to improve efficacy and efficiency during CTO interventions.8–10 All these algorithms agree on the possibility of using all feasible and available techniques and approaches (antegrade, retrograde, true-to-true lumen crossing or re-entry) with the goal of reopening the occluded vessel, maximising the chance of success. Antegrade wiring (AW) is the simplest and most widely used CTO crossing technique and usually represents the first step to learn and master.11 The wire-based techniques, whether antegrade or retrograde, involve the stepwise use of guidewires with increasing stiffness and penetration power. However, during lesion crossing, it is sometimes possible to de-escalate the guidewire’s penetration capacity, for instance, after proximal cap puncture, to navigate more easily and safely within the occlusion. If necessary, re-escalation can then be performed if needed. This has led the Chronic Total Occlusion Academic Research Consortium to recommend abandoning the term ‘wire escalation’ in favour of ‘antegrade wiring’ or ‘retrograde wiring’ (RW), which implies the possibility of both escalation and de-escalation.12
Analysis of Chronic Total Occlusions
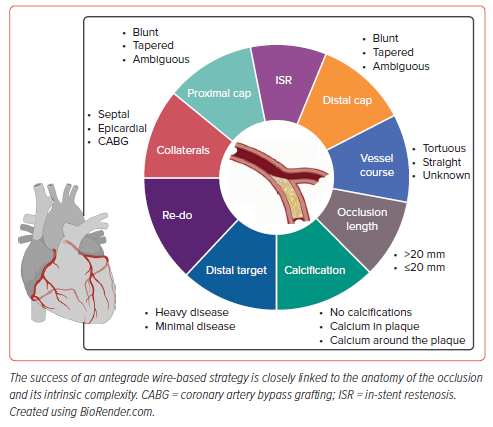
The success of an AW-based strategy is closely linked to the anatomy of the occlusion and its intrinsic complexity (Figure 1). Dual-catheter angiography has a pivotal role during CTO PCI. Almost all current crossing algorithms emphasise the importance of dual-catheter angiography (except for ipsilateral collaterals) and the need for careful review and analysis of the angiography of the target CTO.8,9,10 CTO anatomy dictates the likely most successful and safest crossing strategies, enabling thorough procedural planning as well as the assessment of the risk–benefit profile of the procedure.8,13 To perform an accurate review of the anatomy, a good baseline angiography is needed, which implies the use of wide fields, avoiding panning of the table and using long runs to allow a good visualisation of collateral circulation.
During the angiography analysis, the four most important characteristics to consider are the proximal cap morphology; occlusion length, course and composition (i.e. calcium); the distal target vessel (distal landing zone); and the collateral circulation.
Proximal Cap Morphology
For a successful AW approach, the identification and characterisation of the proximal cap is of paramount importance. The proximal cap can be tapered, blunt or ambiguous. A cap is defined as ambiguous if the exact location of the proximal cap of the occlusion is not clear, because of the presence of side branches or bridging collaterals. Tapered proximal caps are more favourable for wiring than blunt caps, as they provide a channel where the guidewire can be advanced. Blunt proximal caps are usually associated with longer-duration CTOs. The prolonged exposure to systemic arterial pressure causes the formation of dense fibrous tissue, which tends to gradually blunt the cap of the occlusion. However, along with considering the morphology of the cap, other factors must also be considered. For instance, the presence of a bifurcation at the origin of the occlusion may render wiring the proximal cap more challenging due to the prolapse of the wire toward the side branch, which offers a zone of decreased resistance. In these cases, using stiffer and more steerable guidewires is advisable. Alternatively, using a dual-lumen microcatheter, an angulated microcatheter (e.g. SuperCross [Teleflex]), or a steerable microcatheter (e.g. Venture [Teleflex]) to stabilise the guidewire tip can enhance the likelihood of success.
Lesion Length Course and Composition
A dual injection is always necessary to accurately quantify the length of the occlusion: using a single injection greatly increases the likelihood of its overestimation. As the length of the CTO increases, the probability of intraplaque wiring decreases, thereby the possibility of subintimal tracking is higher. This has been demonstrated to be associated with longer procedural and fluoroscopy times, increased use of contrast media and lower procedural success rates.14–16 Therefore, in cases of longer lesions, the likelihood of successfully completing the procedure with AW is very low. In these scenarios, a primary antegrade dissection and re-entry (ADR) or retrograde approach is more suitable and recommended.2,17 The composition of the occlusion body is another important factor to consider. The presence of tortuosity and calcium in the CTO body makes CTO crossing more difficult, increasing the risk of extraplaque tracking and perforation.14 If the lesion is severely calcified, it is predictable and foreseeable that there will likely be a need for guidewires with high penetration power, as well as the possibility of encountering issues because of the lack of backup support.
The Distal Target Vessel
A poor-quality distal vessel in CTO lesions is associated with lower technical and procedural success, a higher risk of complications and longer procedural time.18 Therefore, the evaluation of this parameter is of fundamental importance. When assessing the quality of the distal target, it is important to focus on its dimensions and the presence of atherosclerotic disease. Large (≥2 mm) and healthy distal target vessels are easier to cross and are associated with a higher likelihood of success. It is important to note that the dimensions of the distal portion of a CTO are often underestimated, both because of chronic hypoperfusion and partial visualisation from contralateral injection. Sometimes it is useful to review previous angiographies to obtain a more accurate idea of its dimensions. Additional features to consider include the presence of calcium, which may hamper the feasibility of distal re-entry after an ADR strategy, and the presence of a bifurcation at the location of the distal cap, which could be lost if the re-entry zone falls distal to its origin. Therefore, in cases of a poor-quality distal target or the presence of a bifurcation at the level of the distal cap, a primary retrograde approach can be considered.
Collateral Circulation
While the description of collaterals as interventional collaterals is beyond the scope of this review, the presence and analysis of collaterals as a means of distal visualisation are of paramount importance during an antegrade approach. Collaterals may arise from the same vessel where the CTO is present (ipsilateral collaterals), from another coronary artery (contralateral collaterals) or – in the case of patients who have previously undergone coronary artery bypass grafting (CABG) – from a vascular graft. Regarding the type of collateral vessel based on their location and origin they can be classified as septal, epicardial or grafts. During AW, the use of contralateral visualisation is of utmost importance, as antegrade injections seldom provide valuable insights into wire distal progression and also carry the risk of propagating dissection flaps created by the antegrade manipulation of materials. For the same reason, in the case of ipsilateral collaterals, selective imaging directly through the collaterals using a microcatheter should be considered. The vast majority of the procedure during an antegrade approach should be guided by distal visualisation through contralateral injections, which is why the analysis of collaterals is of paramount importance, even when a retrograde approach is not being considered.
Guidewire Ability
Significant progress has been made in wire technology, allowing for the treatment of more complex CTOs. Guidewire selection and use play a pivotal role during CTO PCI, particularly during a wire-based approach. The selection of the initial wire for AW should be determined by the anatomy of the occlusion. To cross a CTO, the wire should penetrate the proximal cap, navigate through the body of the occlusion, cross the distal cap and finally reach the distal true lumen. It is unlikely that one wire alone can address all the engineering challenges posed by such anatomical heterogeneity, which is why understanding the characteristics of a guidewire is of critical importance. Guidewires consist primarily of four components: the core, the tip, the coating and the cover (Figure 2). Even small variations in these components have a significant impact on the guidewire characteristics. There are several metrics used to evaluate a guidewire performance, with each wire having different capabilities based on the individual characteristics provided by its construction.
Such metrics are as follows:
- Trackability: the ability of a wire to follow the tip down a vessel, particularly when navigating through bends or curves in tortuous vessels. Less stiff, floppy wires can navigate sharp bends much more easily than stiff wires. Trackability is affected by how the tip is designed and the material of the core.
- Steerability: the ability of a wire to be redirected within the lesion and respond effectively to directional changes and navigate through vessels with high precision.
- Torqueability: the ability of a wire to apply rotational force at its proximal end to achieve precise control at the distal end.
- Flexibility: the ability of a wire to flex on its longitudinal axis while maintaining torque and trackability. The flexibility of wires is determined by the material of the core – either very flexible nitinol or stiff stainless steel.
- Crossability: the ability to cross a lesion with minimal or no resistance. Typically, stiffer wires excel in crossing tougher lesions.
- Supportability: the ability of a guidewire to support the passage of another device or system over it.
Guidewire Classification
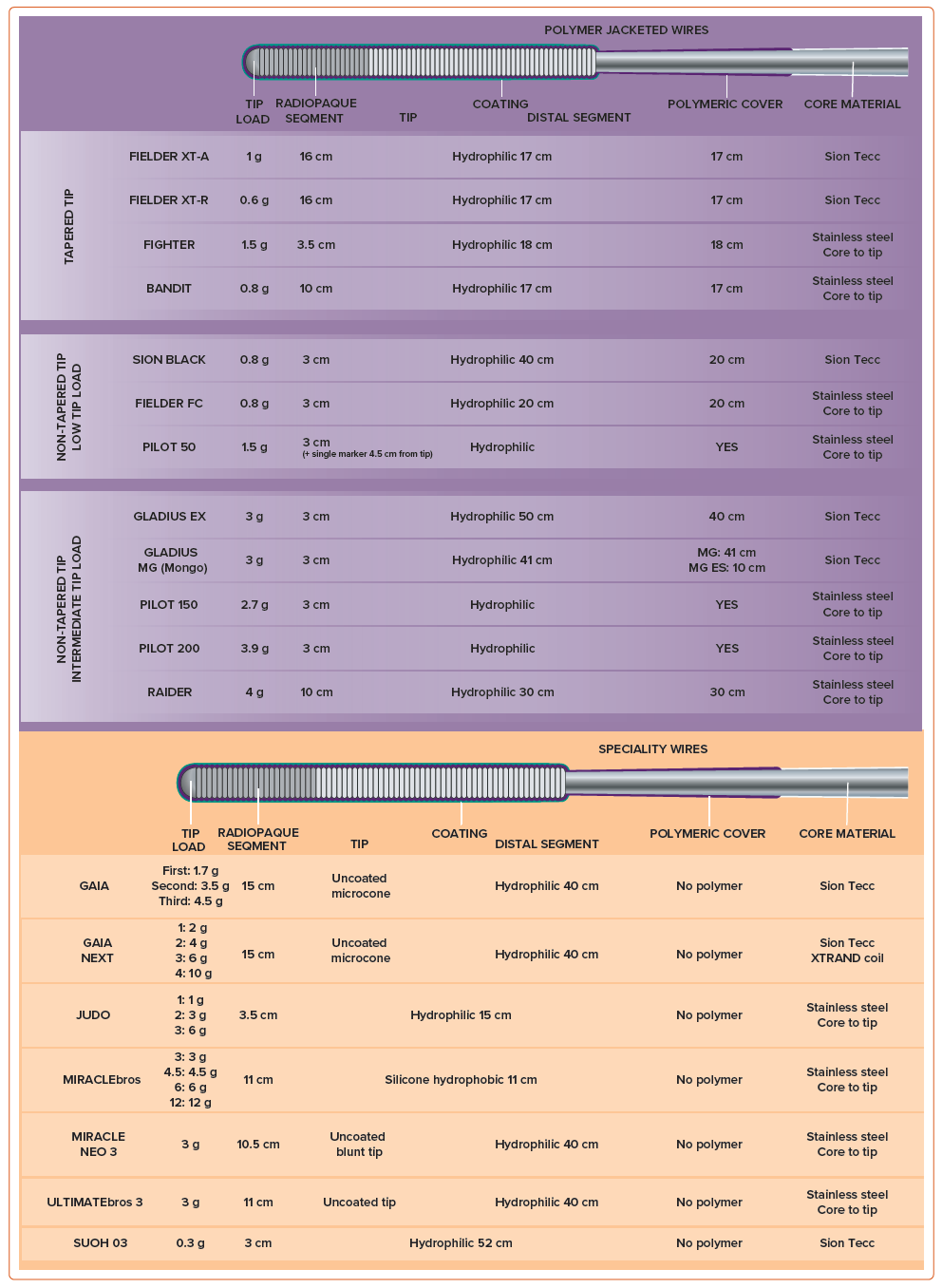
Guidewires used for AW can be divided into three main categories: tapered polymer-jacketed wires, intermediate tip-load guidewires and high tip-load guidewires.
Tapered polymer-jacketed wires are low tip-load wires with tip diameters ranging from 0.008" to 0.010". They are employed for tracking delicate and invisible microchannels. The polymeric cover enhances wire lubricity, facilitating navigation through plaque-dense environments where exposed coil wires may struggle. Because of their low weight and the presence of a distal polymer sleeve, they are rarely associated with vessel exit. Consequently, they are frequently selected as the primary wire option. Tactile feedback is reduced because of the insulation provided by the polymer jacket; therefore, wire advancement should be guided primarily by fluoroscopic feedback. A straight tip progression is the preferred behaviour for the tip of this guidewire family; deflection of the tip must be avoided as it may result in wire entry into the extraplaque space. Sliding is the optimal advancement technique for this category of wires. It involves a gentle tip rotation and probing of the lesion, allowing the guidewire to passively find the path of least resistance, typically through intraplaque microchannels. Examples of wires are the Fielder XT-A or XT-R (Asahi Intecc), Fighter (Boston Scientific) or Bandit (Teleflex).
Intermediate tip-load guidewires encompass a very broad range of guidewires, varying in tip load (between 3 and 6 g), tip diameter (0.008–0.014") and the presence of a polymer cover. The choice of wire should be based on the characteristics of the lesion. Medium gram weight non-tapered polymer-jacketed wires are reliable step-up wires (e.g. Gladius EX or MG [Asahi Intecc], Pilot 200 [Abbott] or Raider [Teleflex]), often used to safely navigate an uncertain vessel course, given their low risk of exiting the vessel architecture. Regarding non-polymer-coated intermediate tip-load guidewires, their selection requires a clear understanding of the vessel course. These guidewires enable active control, penetration and guidance within the lesion. However, they pose a higher risk of perforation because of their penetration power. Steerability is an indispensable characteristic of this category of guidewires.
For non-polymer-jacketed intermediate tip-load guidewires, the best advancement technique is drilling. Drilling consists of controlled, relatively quick rotation movements of the guidewire in both directions by 90°. During drilling, the guidewire is not forcefully pushed into the occlusion; instead, it maintains steady and engaged contact between the plaque and the wire tip. Deflection and rotation technique is a technique specifically employed with Gaia family (Asahi Intecc) guidewires, made possible by their unique and distinctive technology (dual coil and composite core technology). When using this technique, the wire should be advanced in the desired direction. Upon observing a deflection of the tip and the adoption by the wire’s body of a sinusoidal shape, the wire should be retracted and its tip redirected, allowing for controlled advancement of the guidewire and precise navigation through the lesion. Examples of polymer-jacketed intermediate tip-load guidewires group are Gladius EX or MG, Pilot 150/200 or Raider. Examples of non-polymer-jacketed intermediate tip-load guidewires are Ultimate Bros 3 (Asahi Intecc), Judo family (Boston Scientific) or Gaia family.
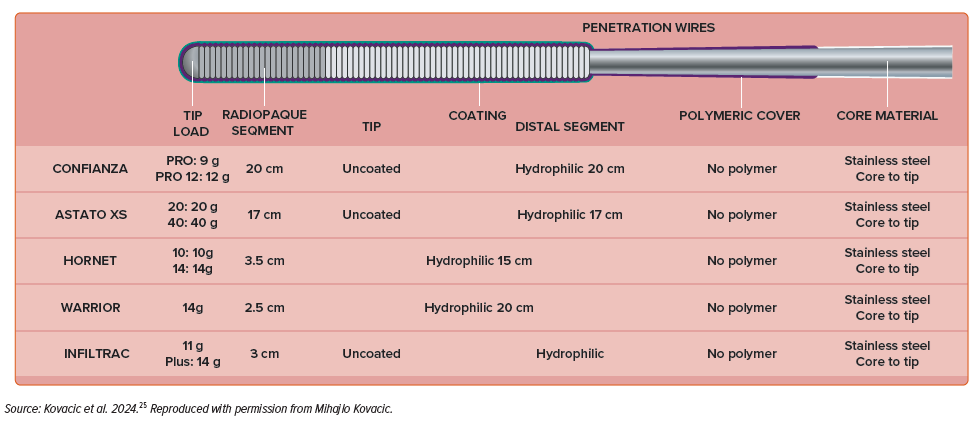
High tip-load guidewires are used to penetrate dense and calcified plaques. They often have a tapered tip to enhance penetration force. However, the increased penetration power comes at the cost of decreased tactile feedback. Their use should be limited to cases where the anatomy is very well defined or when a calcified proximal cap needs to be poked followed by a wire de-escalation.
Penetration is the best advancement technique for these guidewires. This technique involves forward guidewire advancement, using the wire as a ‘needle’ to penetrate the occlusion. Examples of wires are Confianza Pro 12 (Asahi Intecc), Hornet 14 (Boston Scientific), Warrior (Teleflex), Infiltrac and Infiltrac Plus (Abbott) or Astato 20 (Asahi Intecc).
A complete overview of the characteristics of available guidewires is provided in Figure 3.
The Fundamentals of Wire Escalation
Penetration Power
It is important to emphasise that the tip load of a guidewire and its penetration power are two distinct concepts and are not necessarily directly proportional. The penetration power of a guidewire is calculated by dividing the tip weight by the tip surface area. Consequently, tapered guidewires, with a smaller tip area, will have greater penetration power compared with non-tapered guidewires with the same tip load. Additionally, the penetration power of a wire can also be further increased by moving the microcatheter closer to its tip. The stiffness of the wire’s tip changes in an inversely proportional manner to its distance from the microcatheter tip.
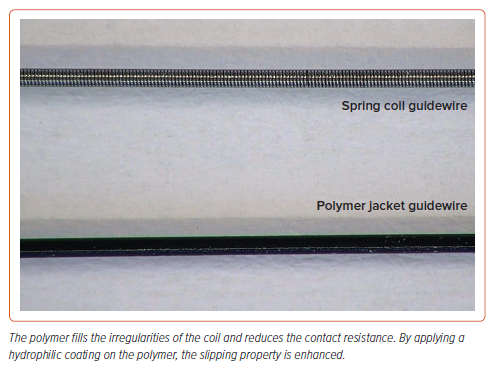
Polymer Cover
The distal segment of polymer-jacketed guidewires is fully covered by a highly slippery polymer (Figure 4). These wires are characterised by high lubricity but low tactile feedback. Polymer-jacketed wires are the preferred choice for tracking small microchannels, but they do not facilitate the tactile distinction between luminal and subintimal wire passage, as well as exhibiting poor directional control. After crossing the occlusion, polymer-jacketed wires may pose a higher risk of complications such as distal vessel perforation and extra-plaque crossing, leading to the creation of dissection planes. Therefore, once the distal true lumen is reached, these wires should be replaced as soon as possible with a standard workhorse guidewire to complete the PCI, delivering equipment such as balloons or stents.
Wire Shape
In CTO PCI, the optimal shape of the wire tip differs from the one typically used in non-CTO PCI procedures, where a standard bend may be sufficient. When dealing with a CTO, the tip shape should be tailored depending on the desired wire function. Typically, a first curve of 1–2 mm with an angle of approximately 40–50° is created at the most distal part of the tip to facilitate the engagement and progression of the guidewire within the proximal cap. Additionally, a second, wider curve (15–20°) may be added to the more proximal part, 3–5 mm from the tip, to enhance manoeuvrability and steerability within the occlusion body. Some guidewires, such as the Gaia, Gaia Next, Gladius and SUOH 03 (Asahi Intecc) might come in a pre-shaped configuration.
Antegrade Wiring
Approaching the Occlusion
The use of a microcatheter is mandatory during CTO PCI. Microcatheters provide support for the guidewire tip, reducing its oscillations within the vessel and enhancing its torque control, as well as increasing penetration power. They also allow for rapid guidewire exchange and reshaping of their tip without losing the acquired position, while simultaneously protecting the proximal vessel segment from potential injury. AW is most likely to be successful when there is a clear, tapered proximal cap, the occlusion is short (<20 mm) and/or the presence of a microchannel is suspected.16,17 If these conditions are not met, the probability of success is lower, but a primary AW strategy may still be appropriate. In this case, operators should have a lower threshold for a switching strategy in the absence of lesion crossing or substantial progress after few attempts.
Once the CTO analysis through the dual injection has been performed, the first step is puncturing the proximal cap. Usually, the first guidewire used for this purpose is a low tip load, tapered, polymer-jacketed guidewire. If the cap is tapered, the use of these guidewires provides a high probability of progression by tracking microchannels. During the advancement, it is important to avoid the crossing of bridging collaterals, which are fragile and often extra-adventitial. Accurate monitoring of the guidewire tip behaviour is essential, as the presence of polymer cover does not provide adequate tactile feedback. If the tip bends, giving the impression of encountering a resistant lesion and thus lacking penetration force, it may be reasonable to escalate the tip load.
A non-tapered polymer-coated guidewire with a higher tip load should be used if the vessel pathway is unclear given the reduced risk of exiting the vascular architecture. Conversely, if the vessel pathway is clear, for instance because of heavy calcifications or vessel straightness, escalating to a non-polymeric guidewire with good steerability, tactile feedback and high penetration power may be preferable. In cases of a blunt proximal cap, which typically presents a composition more resistant to penetration compared with tapered caps, skipping the step of using low-tip load tapered polymeric guidewires may still be appropriate given the anticipated low probability of success.19 In those cases where the proximal cap is not well defined, the use of intravascular ultrasound (IVUS) can help to resolve ambiguity. If there is a sufficiently large side branch to accommodate the IVUS probe adjacent to the occlusion, IVUS can be used both to identify the cap of the occlusion and to perform an IVUS-guided puncture. IVUS can be used either through a ‘live’ IVUS-guided puncture or by intermittent serial imaging. During live IVUS-guided puncture, the probe is positioned at the site where the stump can be best viewed, allowing for continuous monitoring of guidewire motion. This facilitates directing the wire through the occlusion cap, ideally targeting its centre.
To perform this technique, large guide catheters are necessary so that the IVUS catheter and a microcatheter can be simultaneously accommodated: an 8 Fr should be used with large microcatheters (e.g. Corsair Pro [Asahi Intecc] or Turnpike [Teleflex]), while 7 Fr is enough for smaller microcatheters (e.g. FineCross [Terumo] or Caravel [Asahi Intecc]), using a small profile IVUS probe (e.g. OptiCross [Boston Scientific]). When using intermittent IVUS guidance, after location of the proximal cap with the probe, an angiographic acquisition is used to mark when the stump is detected.20
Crossing the Occlusion
Once the proximal cap has been punctured, the guidewire advances into the body of the occlusion. If the lesion does not exhibit evident calcifications, it is rarely necessary to use highly penetrating guidewires with a high tip load. During the advancement through the occlusion, it is essential to pay particular attention to the position of both the guidewire and the microcatheter. If no progress is achieved (typically within a few minutes), cautiously advancing the microcatheter closer to the wire tip can enhance its stiffness. Alternatively, changing the wire can be done without losing the acquired position.
It is crucial to underline the importance of never advancing the microcatheter without first confirming the proper position of the guidewire, as the risk of pushing it outside the vascular architecture could lead to severe consequences. In the majority of cases, a perforation caused by a guidewire does not result in significant complications. However, the scenario is markedly different if the same complication is caused by a microcatheter or other equipment. Guidewire position can be monitored through contralateral injection (two orthogonal views are needed) or fluoroscopic visualisation of the vessel profile, in cases of extensive calcifications or intra-stent lesions. The guidewire should exhibit a synchronous ‘dancing’ movement with the vessel. As previously discussed, antegrade injection is not recommended because of the risk of propagating any dissection flaps created by wiring attempts. Furthermore, often with antegrade crossing techniques, antegrade visualisation can be compromised because of damage to bridging collaterals or microchannels caused by the wire. In the presence of ipsilateral collaterals, a second microcatheter positioned in a donor branch for selective contrast injection can be used, providing a valid means for distal visualisation, while avoiding direct injection from the guiding catheter.
De-escalation
If stiff guidewires were required for puncturing the proximal cap or crossing resistant segments within the occlusion, it is advisable to switch to softer guidewires as soon as possible to reduce the risk of perforation. The characteristics and proprieties of the guidewires during AW must be adjusted throughout the entire procedure based on the type of tissue to cross, with a stepwise process of escalation or de-escalation of their penetration power. The ultimate goal is always to effectively and efficiently reach the distality of the vessel while ensuring safety.
Distal Cap
Compared with the proximal cap, in most cases the distal cap is typically softer and more easily penetrable owing to its limited exposure to systemic pressure. However, in patients with prior CABG, where the distal portion of the occlusion is reperfused by bypass grafts, the distal cap of the occlusion is typically more resistant and challenging to penetrate. In such instances, re-escalating the guidewire may be necessary to puncture the distal cap, thus preventing the guidewire from deflecting towards the extraplaque space and failing to reach the distal true lumen. Once the distal cap is crossed and the distal true lumen is reached, confirmation of guidewire position is mandatory before further advancement of the wire and the microcatheter.
As previously outlined, for this purpose, the use of contralateral injection – performed in two orthogonal projections – is the best method. Alternatively, exchanging for a workhorse guidewire and analysing the behaviour of its tip, along with the tactile feedback provided, can give additional information into the intraluminal position of the guidewire. What should be avoided is performing a tip-injection from the microcatheter. If the equipment lies in the subintimal space, this manoeuvre would generate a hydraulic expansion of the extraplaque space, drastically reducing the chances of re-entry into the true lumen. Once the true lumen is gained with all the equipment, the CTO-crossing guidewire is exchanged with a workhorse guidewire and the procedure can be completed like a conventional PCI.
Troubleshooting
Lack of Back-Up Support
During CTO PCI, crossing the material through the lesion can be challenging, sometimes even leading to the impossibility of advancing the microcatheter or small-profile balloons. This can happen in approximately 6–9% of CTOs.21 During attempts to advance the equipment, the resistance posed by the occlusion to its advancement can cause backward prolapse of the guide catheter, leading to the risk of losing the position of the distal guidewire. Increasing the support is the first step in this scenario. This can be addressed by several measures, including anchor strategies, the use of higher support microcatheters or guide catheter extension (GCE).
Anchor Strategies
A workhorse guidewire is advanced into a proximal side branch (for instance a conus or acute marginal branch for the right coronary artery or a diagonal for the left anterior descending artery) followed by the delivery of a small balloon (sized 1:1) inflated at nominal pressure. It should be noted that the use of this technique may result in the injury or dissection of the side branch, although usually without any significant complications.
Higher Support Microcatheters
Coiled and torquable microcatheters are generally more supportive than braided ones. The use of a microcatheter with a tapered tip is often useful to engage the lesion by wedging and making space within it. There are also devices known as plaque-modification microcatheters, which are highly supportive devices specifically designed for advancing through calcified and difficult-to-penetrate lesions, such as the Tornus (Asahi Intecc) and the Turnpike Gold (Teleflex).
Guide Catheter Extension
A GCE can help to improve guide catheter support and pushability of the antegrade equipment by increasing the amount of coaxial force that can be delivered.22 Additionally, the use of a GCE has been demonstrated to be more effective than conventional techniques, such as buddy wire or anchoring balloon, in facilitating the success of transradial PCI for complex coronary lesions.23
Extraplaque Guidewire Position
If the antegrade wire ends up in the extraplaque space, this does not imply that the AW strategy has failed or that a different approach is needed. Several techniques have been developed over the years to address these scenarios. One such technique is wire redirection, whereby the guidewire is retracted and redirected. This can be performed only when a steerable guidewire is used, such as the Gaia and Gaia Next family.
Another such technique involves the use of a parallel wire, whereby, after leaving the first guidewire in place, a second guidewire (usually stiffer and more steerable) is advanced in ‘parallel’ to the first one. The key concept of this technique is to close the entry point to the subintimal space with the first wire, preventing the second one from tracking the wrong pathway and enhancing the possibility of following a new route through the intraplaque space. Usually, in this technique the microcatheter is removed from the first wire and reused during the new attempt. Several variations of this technique have been described, the ‘see-saw’ technique that involves the use of a second microcatheter in parallel to the first one or the dual-lumen microcatheter-facilitated parallel wire.24
If the wire remains subintimal, switching to an ADR or a retrograde approach should be considered. Operators not able to perform a retrograde approach or ADR should stop the procedure and refer the patient to a dedicated CTO centre.
Retrograde Wiring
RW is the least frequently employed technique in CTO PCI and its success rate is very low – approximately 10% of cases.1 This strategy, in contexts such as the presence of an ambiguous proximal cap, can be chosen as a primary approach, particularly in cases of occlusions where the anticipated probability of success through the RW of the occlusion is high (short, non-calcified lesions). As with the antegrade approach, the choice of guidewire is dictated by the anatomy of the distal cap and the characteristics of the lesion. When using injection from the donor vessel, it is often challenging to precisely characterise the position and morphology of the distal cap. In such cases, distal tip injection from the retrograde microcatheter can be useful before the wiring attempt. As discussed previously, the distal cap of an occlusion is usually tapered and less resistant compared with the proximal one, because it is not chronically exposed to systemic pressure (except in patients who have undergone CABG). Consequently, the need for guidewires with high penetration power to puncture these stumps is rare. If the distal cap presents substantial resistance to wire crossing, a penetration wire may be necessary. In such cases, particular attention should be paid to de-escalating and exchanging these guidewires as soon as possible, as retrograde perforations can be very challenging to control. The procedural steps and general concepts used for puncturing the proximal cap, navigating through the occlusion and accessing the true distal lumen from a retrograde approach are the same as those already described in the above paragraphs. However, discussions regarding what happens after crossing the CTO with a retrograde guidewire, such as the externalisation process or converting the system to an antegrade approach (tip-in or rendezvous techniques), are beyond the scope of this review.
Conclusion
Advances in technology and techniques continue to enhance efficacy and patient care in CTO management. A deep understanding of available materials, techniques and crossing algorithms is crucial when starting a CTO program, as this can significantly improve procedural success. Careful angiography analysis combined with dual catheter injection is essential before the procedure, providing important information for a successful procedural plan. AW is the preferred and primary crossing technique for tapered and short lesions, and it is typically the first approach to master. The use of RW is less frequent in CTO PCI because of its lower success rate. However, fundamental concepts in lesion approach and crossing remain consistent in both cases. It is unlikely that a single approach alone can adequately address all challenges posed by anatomical CTO heterogeneity. CTO operators should strive to enhance and master all available techniques and approaches, thereby substantially increasing their success rate.